A. Lindauer1, R. Croff1, K. Mincks1, N. Mattek1, S.J. Shofner2, N. Bouranis3, L. Teri4
1. Oregon Health & Science University, Portland, Oregon, Layton Aging and Alzheimer’s Disease Center, USA; 2. Portland State University, Portland, Oregon, USA; 3. Oregon Health & Science University-Portland State University School of Public Health; 4. University of Washington, School of Nursing & Northwest Roybal Center; Northwest Research Group on Aging
Corresponding to: Allison Lindauer, PhD, NP, Assistant Professor, Layton Aging and Alzheimer’s Disease Center, Director, Outreach, Recruitment & Education, Assistant Professor, OHSU School of Nursing, 3181 SW Sam Jackson Park Road, CR131; Portland, Oregon 97239, USA, Tel: 503-494-6976| Fax: 503-494-7499; lindauer@ohsu.edu
Care Weekly 2018;2:25-31
Published online July 3, 2018, http://dx.doi.org/10.14283/cw.2018.4
Abstract
Background: Caring for a family member with Alzheimer’s disease and related dementias can be mentally and physically taxing. Support programs are available to mitigate the strain of care, but caregivers report access challenges (e.g., distance). STAR-C is an evidence-based, effective, one-on-one caregiver educational intervention. However, family caregivers who do not live near a STAR-C consultant (e.g., rural caregivers) cannot participate in the program. The earth-bound mode presents a critical barrier to widely-available caregiver support.
Objectives: We assessed the feasibility, preliminary efficacy, and cost of implementing a caregiver support intervention (STAR-C-Telemedicine), using Internet-based videoconferencing.
Design: Using a mixed-methods approach, we examined feasibility and pre- and post-intervention changes in caregiver burden. Focus groups provided feedback on program acceptability.
Setting: Participants, in their own homes, connected the university-based study staff using videoconferencing technology.
Participants: Twenty family caregivers for those with dementia consented to the study.
Intervention: The STAR-C-TM intervention included 8 weekly sessions in which the university-based consultant met (via videoconferencing) with caregivers in their homes. The intervention focused on identifying upsetting behaviors and identifying triggers to the behaviors.
Measurements: We assessed caregiver burden, depression and desire to institutionalize prior to and after the intervention.
Results: Fourteen caregivers (82% of those who started the intervention) completed all study components. We found statistically significant reductions in caregiver burden. Caregivers liked the videoconferencing option. Almost two-thirds reported, given the choice, that they would prefer it over an in-person offering. STAR-C-TM saved, on average, $1150/per caregiver over the traditional program. Qualitative findings supported the quantitative data.
Conclusions: Telemedicine-based support for family caregivers is a feasible and cost-effective option. As the prevalence of dementia grows, programs such as STAR-C-TM can fill an important gap in caregiver education and support.
Key words: Caregivers, dementia, telemedicine.
Introduction
Alzheimer’s disease and related dementias (ADRD) affect almost 47 million people around the globe, with worldwide costs approaching 1 trillion dollars (US) in 2018 (1). ADRD is emotionally taxing as well (2). As those with dementia develop memory loss and behavioral symptoms, their family caregivers often experience emotional, physical and financial strain (2, 3).
The hallmark symptom of ADRD, a group of progressive neurodegenerative diseases with no cure, is cognitive impairment (4). However, it is the behavioral symptoms (e.g., irritability, pacing, medication refusal) that predict caregiver burden and depression (5, 6). While we wait for a cure, efforts to contain these emotional and financial costs should focus on supporting the health and well-being of all living with ADRD, including the family caregivers. Telemedicine-based support and education has the potential to curb these costs for any caregiver with a computer and Internet access.
Evidence-based programs for caregivers are available to sustain their emotional health by easing burden and ameliorating depressive symptoms through education (7). However, caregivers report multiple barriers to participation, including inconvenient meeting times, lack of respite care for the person with ADRD, lengthy travel time and stigma (8, 9).
With the aim of improving access to caregiver support, interventionists have turned to technology. Internet chat rooms, self-paced programs, and videoconferencing programs are options discussed in the literature (10-13). Many of these telemedicine-based interventions are group-based, yet the evidence suggests that individualized interventions are more effective in reducing caregiver distress (7). Few of the telemedicine-based opportunities reported in the literature are one-to-one options that take advantage of the social connection permitted by face-to-face videoconferencing (10). More personalized choices are needed to fit the heterogenic needs of family caregivers. STAR-C is an example of such an intervention, and it is well-suited for translation into a direct-to-home telemedicine program (14).
STAR-C is an evidence-based program that teaches family caregivers how to manage behavioral disturbances in family members with ADRD, with the goal of reducing behavioral symptoms and, in turn, caregiver burden (14, 15). STAR-C significantly reduces caregiver burden and depression, and improves quality of life for those with ADRD.(14, 15). STAR-C uses “consultants,” health care providers (e.g., nurses & social workers), who meet one-on-one with caregivers in their homes. However, this in-home training is not available for those who do not live near a consultant.
To increase caregiver access to STAR-C, we developed and tested STAR-C-Telemedicine (TM). Our exploratory study had three aims: (1) Examine feasibility, cost, and consumer satisfaction with STAR-C-TM (2), compare scores on measures of caregiver depression and burden before and after the STAR-C-TM intervention (3), explore effect of STAR-C-TM training on LTC placement plans for care recipients.
Methods
Subjects
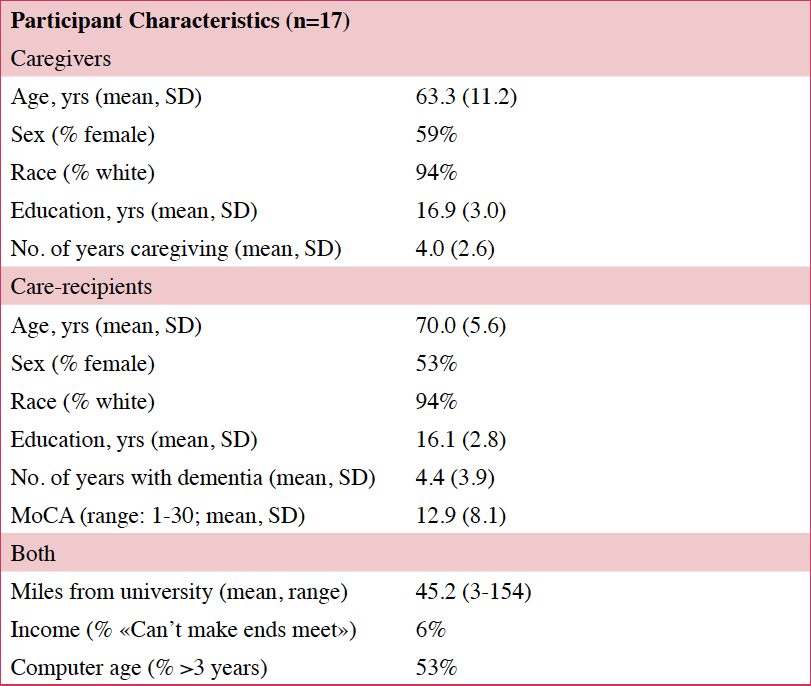
Twenty caregivers for persons with ADRD consented to participate in the study. All study activities were approved by the OHSU IRB (#15695). Caregivers were recruited from the OHSU Aging and Alzheimer’s Disease Clinic and community organizations. Caregivers were over age 18 and spent at least four hours/day caregiving. All care recipients had a diagnosis of ADRD, which includes Alzheimer’s disease (AD), Lewy Body disease, and vascular dementia. Care recipients exhibited three or more behaviors that were upsetting to the caregiver (e.g., pacing, yelling, medication refusal). Both caregivers and care recipients consented to the study, via telephone, but if care recipients did not have capacity to consent, caregivers consented for them, per our IRB-approved policy.
Participants used computers or tablets with Internet connectivity to access STAR-C-TM. We provided iPads for those who did not have computers or strong broadband connections. The two iPads were programmed by the OHSU Telehealth Department, and only the study could be accessed. We mailed the iPads to participants with return packaging and postage; all iPads were successfully returned.
Procedures
After consenting to the study, participants were asked to complete five components that made up the study protocol: (a) technological set-up, (b) pre-intervention assessment, (c) STAR-C TM intervention, (d) post-intervention assessment, and (e) an optional post-intervention focus group.
The Research Assistant (RA, KM) provided the videoconferencing link and coached caregivers on downloading and using the link. She assisted caregivers with technical challenges throughout the participation period. After the videoconferencing was set up, the RA administered assessments via telemedicine directly to participants in their homes (see Measures).
The PI, an advanced practice nurse with a PhD in gerontological nursing, was the consultant in this pilot. She provided 8 weekly, hour-long, direct-to-home sessions, via videoconferencing, to the caregivers per the original STAR-C protocol (Table 1). Caregivers received a booklet, Understanding Alzheimer’s and weekly handouts. If the caregivers agreed, the sessions were videotaped for quality assessment and training. The consultant telephoned the caregivers at 1 and 2 months after the 8 sessions to review material and address additional concerns.
As with the original face-to-face intervention, the STAR-C-TM consultant and caregiver sessions were private. Care recipients had to be engaged in a separate activity (e.g., taking a nap, watching television) in another room while caregivers participated in the training sessions.
The post-assessment was administered within a week of completion of the 8-week sessions, then two months after the last intervention session. The post-intervention assessments were identical, except for the pre-intervention demographic survey and a user satisfaction survey used on the final assessment (16).
Caregivers who completed all the above elements were asked to participate in an optional focus group. Two focus groups (total of 7 caregivers) were conducted via videoconferencing. An independent scientist, (RC) moderated the groups.
Measures
We assessed feasibility and participant satisfaction with two surveys and two focus groups (16, 17). Caregiver burden was assessed with the 24-item Revised Memory and Behavior Problems Checklist (RMBPC) (18), and the Screen for Caregiver Burden (SCB) (19). The RMBPC and has excellent reliability when used with videoconferencing technology (ICC=0.80)(20).
The Desire to Institutionalize (DTI) scale (21) includes 5 dichotomous items and one modified item that rates the likelihood of placement on a 5-point Likert scale (1, “not at all likely” to 5, “very likely”). Caregiver depressive symptoms were measured with the Center for Epidemiologic Studies Depression Scale (CES-D) (22).
To characterize cognitive impairment in care recipients, the Montreal Cognitive Assessment (MoCA) was administered via videoconferencing, prior to the caregiver intervention. The MoCA is a 30-item tool used to assess cognitive impairment and is reliable when used with telemedicine videoconferencing (ICC=0.93) (20, 23). After the MoCA, care recipients no longer participated in the study and did not have any further testing.
We compared the cost of providing STAR-C-TM to the traditional, in-person STAR-C. We calculated the theoretical costs of travel, fuel, and consultant time for each family if we had to travel to their homes to administer the STAR-C intervention.
Data Analysis
Descriptive statistics were used to describe the sample. Feasibility was assessed quantitatively by calculating the proportion of participants who completed the full intervention to the total number who enrolled and completed the first assessment. Consumer satisfaction and feasibility was assessed qualitatively via focus group feedback. The focus groups were recorded, and the resulting transcriptions were analyzed using constant comparative analysis techniques with an interpretive phenomenological lens. Interpretive phenomenology seeks to identify the meanings people ascribe to daily phenomena (24). We used the online Dedoose program (https://www.dedoose.com) to facilitate our analysis.
Preliminary efficacy was assessed by comparing the outcome measures before and after the intervention using paired t tests (25). DTI scores were calculated. STAR-C-TM costs were compared to traditional STAR-C costs.
Results
Participants
Twenty caregiver/care recipient dyads consented to the study; 14 completed all study components (Table 2). Three participants consented but did not complete any assessments. Another three participants started the intervention but withdrew before completing the protocol. None withdrew due to technical difficulties. There were no differences in age or education of the caregivers or care recipients of those who withdrew (n=3) compared those who completed study. There were no differences in age, education or comfort with computer use between the caregivers who participated in the focus groups (n=7) compared those who did not.
Feasibility/Ease of Use (Aim 1)
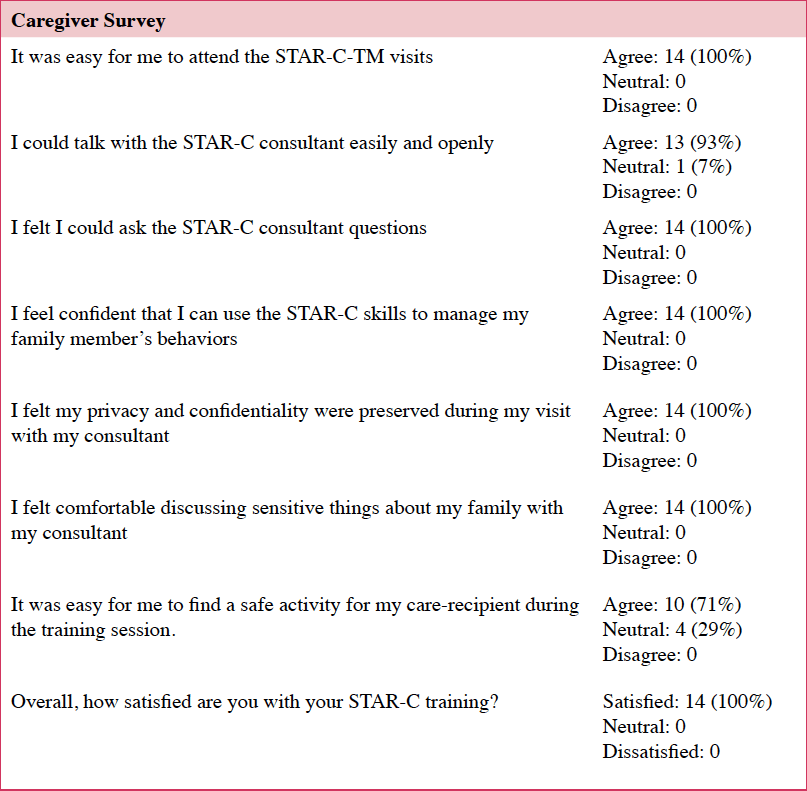
Of those that started the intervention (n=17), 82% (n=14) completed all eight sessions and assessments. Caregivers who completed the intervention found it easy to attend STAR-C-TM visits and all 14 caregivers agreed that their privacy was protected. Finding an activity for the care recipient during the sessions was somewhat challenging, with one in three having some difficulty (Table 3).
There were some technological issues (e.g., pixilation, dropped calls, sound distortion), however, these were interruptions that did not affect the delivery of the intervention. Some of the caregivers were unfamiliar with technology and step-by-step instructions, by our RA, were needed. None of the caregivers dropped out due to connectivity issues.
Qualitative data from the focus groups rounded out the quantitative findings by providing information about the meaning STAR-C-TM held for the caregivers. Overall, STAR-C-TM meant increased access for caregivers: “This was a godsend to me. I live in [a rural area]; it’s a ways to get up there. This was fantastic…I can’t say enough good things about it, to tell you the truth.” Caregivers felt that STAR-C-TM “…took the stress out of getting help.”
One caregiver expressed frustration with videoconferencing: “I think I had a few more technical challenges. It’s not that I’m not tech savvy, but the audio stuff, my head phones weren’t working right… it was… kind of annoying sometimes… But once everything was set up it really did feel very conversational and very comfortable. I didn’t feel like it was weird to be having a conversation via computer…”
Another caregiver was concerned that the videoconferencing sessions without her care recipient felt clandestine (even though care recipients consented to the study); she felt she was “telling secrets” about him. On the other hand, some caregivers felt it was easier to have the STAR-C-TM sessions in their homes so they could keep an eye on their care recipient while engaged in the sessions: “…it’s that tension of feeling like you’re at home and you need to be available, but you also want some privacy to have these conversations.”
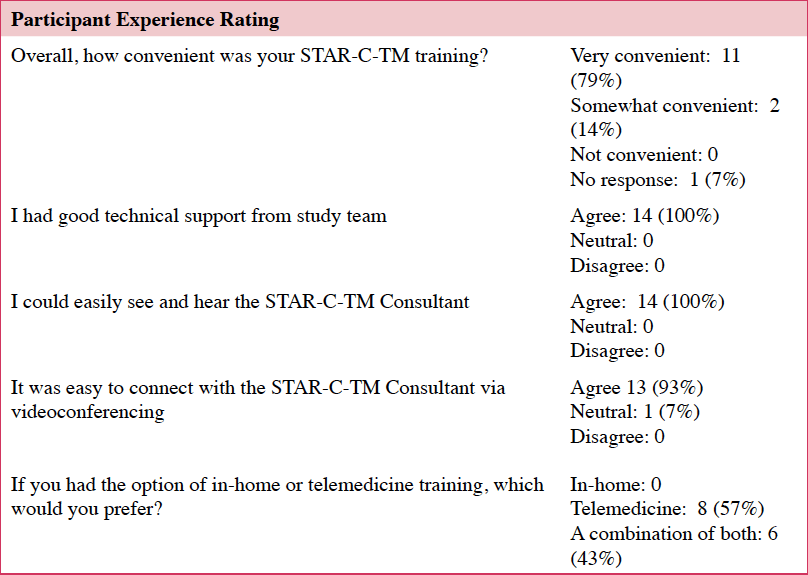
Most participants felt that the videoconferencing was easier than an in-person option: “…I thought this format maybe was better than almost a face to face…It was very focused. It was very intimate. And it was very substantive.” This aligns with the quantitative data in which almost two thirds of the participants preferred the telemedicine option only, fewer than half would have preferred a combination of in-home and telemedicine, and none preferred in-home STAR-C over the telemedicine option (Table 4).
When asked what was most problematic about STAR-C-TM one caregiver replied: “Well, the fact that it was over.” While caregivers liked the one-on-one sessions, some felt there could be additional group work to decrease isolation: “I wouldn’t want to give up the individual. I’m greedy. If I can have some group sessions in addition…that would be delightful…”
Our last feasibility assessment was cost. STAR-C-TM saved, on average, $1150/person over traditional, in-person STAR-C. This was due to the elimination of travel and fuel costs.
Efficacy (Aim 2)
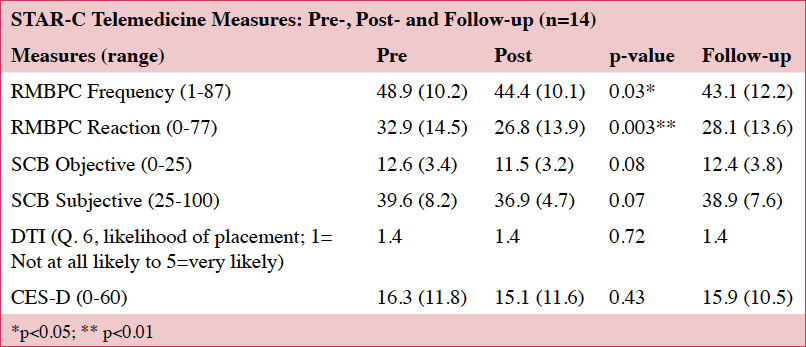
We saw decreases in the frequency of upsetting behaviors and reductions in the caregivers’ reactivity to the behaviors on the RMBPC.(18) No significant changes were noted on the CES-D (22) or the SCD (19) (Table 5). These findings are based on a small sample size (n=14) and lack sufficient power for identifying any important effects of the intervention.
Desire to Institutionalize (Aim 3)
No significant changes were noted on the DTI. For the 14 that completed the study protocol, one caregiver noted she would possibly place and another reported she was “very likely” to place at the final assessment. These two caregivers had lower (better) scores on the CESD (22) and the RMBPC (18) than the sample average, but their care recipients had lower (worse) scores on the MoCA (23) than the group average.
Discussion
Our pilot findings suggest that it is feasible to administer STAR-C-TM directly to caregivers’ homes using telemedicine-based videoconferencing, and the caregivers liked the telemedicine-based option (Aim 1). STAR-C-TM reduced the behavioral symptoms of dementia and caregiver reactivity to them (Aim 2), but STAR-C-TM did not reduce the caregivers’ considerations for long term placement (Aim 3).
For Aim 1, our findings mirrored McCurry et al.’s (2015) in which most participants completed the all 8 sessions. The percentage of caregivers who completed our intervention (82%) was lower than Teri et al.’s (2005) (89%), but higher than McCurry et al.’s (2015) Oregon-based study (64%). The high completion rates and strong consumer acceptance may be due to three qualities of STAR-C-TM: It was possible, private and personalized.
First, it was possible for the caregivers and consultant to engage in STAR-C-TM because the videoconferencing option was available online and inexpensive. Oregon ranks 4th in the nation for broadband coverage, making the telemedicine mode of care an accessible option for many.(26) Arguably, broadband coverage is limited in some areas, which is an obstacle to widespread implementation of programs like STAR-C-TM. Yet, this trend is shifting, indicating that broadband coverage is no longer a luxury but a needed utility.
Second, privacy was a “taken for granted” (24) benefit of STAR-C-TM. All visits were conducted via a HIPPA-compliant videoconferencing link, but what the caregivers seemed to appreciate was that the consultant could only see them and little else. This liberated them from having to conform to social mores, such as cleaning up the house (“You don’t see the mess behind me”) or making coffee for the consultant. Some caregivers felt that having a stranger (the consultant) visit their home would have caused emotional stress, such as anxiety or suspiciousness on the care recipient’s part. Further, consultant safety concerns (e.g., car travel, unfriendly dogs, unsafe neighborhoods) were non-existent. On the other hand, there is value in seeing the full home environment and the telemedicine option did not allow this. Despite the limited view, the caregivers reported feeling that the consultant cared about them and that the intervention was tailored to their needs.
The STAR-C interventions are customized to the needs of each family, (14, 15) and the STAR-C-TM caregivers liked this personalized approach with the one-on-one mode: “I like the focus…we’re not talking about everybody else.” Further, not all caregivers prefer the group setting.(27) The literature points to two phenomena that may interfere with group participation: stigma by association and social anxiety. Werner et al. (2008) found that caregivers experience stigma by association due to care recipient behaviors, and this stigma can curb caregivers’ desire to seek support. Social anxiety may also play a role, with over 5% of older adults having clinically significant social anxiety.(29) Thus, the one-on-one STAR-C-TM option may be alternative for those who feel uncomfortable, for whatever reason, in the group environment.
Caregivers reported feeling emotionally supported within the technological interface. Empathetic engagement is an important concept in healthcare in that it promotes patient engagement. This “digital empathy”(30) was evident within the STAR-C-TM intervention as this caregiver remarked about the consultant: “I felt like I had a really good friend, and I really appreciated that.” Of interest, many of the final STAR-C-TM sessions ended with a “digital hug” in which both the caregiver and the consultant mimed a hug.
Regarding Aim 2 (efficacy), we found significant changes in the frequency and reactivity to care-recipient behaviors, but no change in levels of depression. In contrast, traditional STAR-C interventions have shown improvements in depression.(14, 15) This may be due to our small sample size, and the fact that all telemedicine-administered post-tests were completed with 14 caregivers, thus capturing all the data. This diverges from other community-based STAR-C interventions in which not all post-tests were mailed back.(14, 15)
We did not find any significant changes in the caregivers’ desire to place care recipients in long term care (Aim 3). These findings are similar to McCurry et al.’s (2015) who found no significant change in caregiver’s likelihood of placing their care recipients after receiving the traditional STAR-C intervention. The lack of change in our study may have been due to low levels of desire to place at the beginning of the intervention. While the decision to place is multi-faceted, STAR-C-TM may have paradoxically provided support for this decision because it addresses caregiver health and encourages caregivers to seek respite care when needed.
As a pilot, there were limitations. With the small sample size, it was not powered to generalize findings. We did not do a post-intervention assessment of those who dropped out of the study, so we don’t know what these caregivers thought about the feasibility of the study.
We did not control for external support group participation because we wanted the STAR-C-TM intervention to occur pragmatically, within the day-to-day experiences of caregivers. Additionally, some caregivers’ care recipients were clinic patients of the consultant, which could have influenced our findings.
Despite these limitations, our findings in this pilot study indicate that providing the STAR-C intervention via telemedicine is acceptable and beneficial for caregivers for those with ADRD. The benefits of pilot studies are that they prime us for more elegant, larger studies. This was indeed the case for STAR-C-TM, which laid the foundation for future work to assess fidelity and efficacy. Our ultimate goal is to make this program available to any caregiver with a computer an Internet connection.
Funding
This study was funded by the National Institute on Aging (P30AG008017 & P30AG024978). Funding was also provided by the BUILD EXITO program. Research reported in this publication was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health Award Number UL1GM118964. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Direct all correspondence to Dr. Lindauer: lindauer@ohsu.edu
Conflict of Interest
None.
References
1. Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jonsson L, Liu Z, Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1-7. Epub 2016/09/02. doi: 10.1016/j.jalz.2016.07.150. PubMed PMID: 27583652; PMCID: PMC5232417.
2. Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Annals of Internal Medicine. 2015;163(10):729-36. doi: 10.7326/M15-0381.
3. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. International Psychogeriatrics / IPA,. 2014;26(5):725-47. doi: 10.1017/S1041610214000039.
4. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association,. 2011;7(3):263-9. doi: 10.1016/j.jalz.2011.03.005.
5. Cuijpers P. Depressive disorders in caregivers of dementia patients: A systematic review. Aging & Mental Health. 2005;9(4):325-30. doi: H77P0180460X0QGH.
6. van der Lee J, Bakker TJEM, Duivenvoorden HJ, Dröes R-M. Multivariate models of subjective caregiver burden in dementia: A systematic review. Ageing Research Reviews. 2014;15:76-93. doi: http://dx.doi.org.liboff.ohsu.edu/10.1016/j.arr.2014.03.003.
7. Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders,. 2007;101(1-3):75-89. doi: 10.1016/j.jad.2006.10.025.
8. Karlin NJ, Bell PA, Noah JL, Martichuski DK, Knight BL. Assessing Alzheimer’s support group participation: A retrospective follow-up. American Journal of Alzheimer’s Disease. 1999;14(6):326-33. doi:10.1177/153331759901400607
9. Brodaty H, Thomson C, Thompson C, Fine M. Why caregivers of people with dementia and memory loss don’t use services. Int J Geriatr Psychiatry. 2005;20(6):537-46. doi: 10.1002/gps.1322. PubMed PMID: 15920707.
10. Griffiths L, Blignault I, Yellowlees P. Telemedicine as a means of delivering cognitive-behavioural therapy to rural and remote mental health clients. Journal of Telemedicine and Telecare,. 2006;12(3):136-40. doi: 10.1258/135763306776738567.
11. Austrom MG, Geros KN, Hemmerlein K, McGuire SM, Gao S, Brown SA, Callahan CM, Clark DO. Use of a multiparty web based videoconference support group for family caregivers: Innovative practice. Dementia. 2015;14(5):682-90. doi: 10.1177/1471301214544338.
12. Scott JL, Dawkins S, Quinn MG, Sanderson K, Elliott KE, Stirling C, Schuz B, Robinson A. Caring for the carer: A systematic review of pure technology-based cognitive behavioral therapy (TB-CBT) interventions for dementia carers. Aging Ment Health. 2016;20(8):793-803. doi: 10.1080/13607863.2015.1040724. PubMed PMID: 25978672.
13. Parker Oliver D, Patil S, Benson JJ, Gage A, Washington K, Kruse RL, Demiris G. The Effect of Internet Group Support for Caregivers on Social Support, Self-Efficacy, and Caregiver Burden: A Meta-Analysis. Telemed J E Health. 2017;23(8):621-9. Epub 2017/03/23. doi: 10.1089/tmj.2016.0183. PubMed PMID: 28328392.
14. Teri L, McCurry SM, Logsdon R, Gibbons LE. Training community consultants to help family members improve dementia care: A randomized controlled trial. The Gerontologist. 2005;45(6):802-11. doi: /10.1093/geront/45.6.802.
15. McCurry SM, Logsdon RG, Mead J, Pike KC, La Fazia DM, Stevens L, Teri L. Adopting evidence-based caregiver training programs in the real world: Outcomes and lessons learned from the STAR-C Oregon Translation Study. Journal of Applied Gerontology : The Official Journal of the Southern Gerontological Society,. 2015. Epub 2015. doi: 0733464815581483.
16. Chang H. Evaluation framework for telemedicine using the logical framework approach and a fishbone diagram. Healthcare Informatics Research,. 2015;21(4):230-8. doi: 10.4258/hir.2015.21.4.230.
17. Poulsen KA, Millen CM, Lakshman UI, Buttner PG, Roberts LJ. Satisfaction with rural rheumatology telemedicine service. International Journal of Rheumatic Diseases. 2015;18(3):304-14. doi: 10.1111/1756-185X.12491.
18. Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging. 1992;7(4):622-31. doi: 10.1037//0882-7974.7.4.622. PubMed PMID: 1466831.
19. Vitaliano PP, Russo J, Young HM, Becker J, Maiuro RD. The Screen for Caregiver Burden. The Gerontologist. 1991;31(1):76-83. doi: 10.1093/geront/31.1.76.
20. Lindauer A, Seelye, A., Lyons, B., Dodge, H.H., Mattek, N., Mincks,K., Kaye, J., Erten-Lyons, D. Dementia care comes home: Patient and caregiver assessment via telemedicine. The Gerontologist. 2017;Oct(57(5):e85-e93). doi: 10.1093/geront/gnw206.
21. Morycz RK. Caregiving strain and the desire to institutionalize family members with Alzheimer’s disease. Possible predictors and model development. Research on Aging. 1985;7(3):329-61. doi: 10.1177/0164027585007003002.
22. Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385-401. doi: 10.1177/014662167700100306.
23. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695-9. doi: 10.1111/j.1532-5415.2005.53221.x.
24. Benner P. The tradition and skill of interpretive phenomenology in studying health, illness, and caring practices. In: Benner P, editor. Interpretive phenomenology: Embodiment, caring, and ethics in health and illness. Thousand Oaks, CA: Sage; 1994. p. 99-127.
25. Munro BH. Statisical Methods for Health Care Research. 5th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2005.
26. Strategic Networks Group RT. The 50 States of Broadband. 2017.
27. Wahbeh H, Svalina MN, Oken BS. Group, one-on-one, or internet? Preferences for mindfulness meditation delivery format and their predictors. Open Med J. 2014;1:66-74. doi: 10.2174/1874220301401010066. PubMed PMID: 27057260; PMCID: PMC4820831.
28. Werner P, Heinik J. Stigma by association and Alzheimer’s disease. Aging & Mental Health. 2008;12(1):92-9.
29. Karlsson B, Sigström, R., Östling,S., Waern, M., Börjesson-Hanson, A.,Skoog, I. . DSM-IV and DSM-5 prevalence of social anxiety disorder in a population sample of older people. Am J Geriatr Psychiatry. 2016;24:1237-45.
30. Terry C, Cain J. The emerging issue of digital empathy. American Journal of Pharmaceutical Education. 2016;80(4):58. doi: 10.5688/ajpe80458 [doi].