Li Hongyan1, Lu Wanting2, Li Fei1
1. General Surgery Department of Xuanwu Hospital of Capital Medical University, Beijing City 100000 PRC; 2. Department of Neurology of Xuanwu Hospital of Capital Medical University, Beijing City 100000 PRC
Corresponding to: Li Hongyan, General Surgery Department of Xuanwu Hospital of Capital Medical University, Beijing City 100000 PRC, Email:hongyanli09@126.com; Phone:18600346925
Care Weekly 2021;5:1-5
Published online April 28, 2021, http://dx.doi.org/10.14283/cw.2021.1
Abstract
Purpose: To evaluate mental health, cognitive function, and living quality of colon and rectal carcinoma patients with oxaliplatin-induced neurotoxicity. Methods: Fifty recurrence-free colorectal cancer (CRC) patients with oxaliplatin chemotherapy while 50 control patients without oxaliplatin chemotherapy were enrolled in this study. Subjective and objective aspects of oxaliplatin chemotherapy symptoms were assessed with oxaliplatin neurotoxicity classification. Psychological assessment was measured via the Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS). Cognitive function was measured via Montreal Cognitive Assessment (MoCA). Quality of Life (QOL) was assessed using the World Health Organization’s Quality of Life (WHOQOL-BREF) shortened instrument. Results: Of the patients with oxaliplatin chemotherapy, 41 patients had depression and 42 patients had anxiety. Patients with oxaliplatin chemotherapy scored higher on average on both the SDS (64.36 ± 7.22) and SAS (67.49 ± 9.41) compared to those without oxaliplatin chemotherapy (SDS, 57.86 ± 5.27, p=0.006; SAS, 61.57 ± 10.06, p = 0.004). Patients with oxaliplatin chemotherapy, on average, scored lower on the MoCA (23.46 ± 3.17) compared to patients without oxaliplatin chemotherapy (27.49 ± 2.03, p < 0.05). In addition, patients with oxaliplatin chemotherapy scored significantly lower on measures of physical health (18.9 ± 7.8 vs. 37.8 ± 6.2, p<0.05), psychological health (19.3 ± 8.2vs. 39.8 ± 8.1, p<0.05), and social relationship (50.2 ± 10.1 vs. 70.6 ± 10.5, p<0.05) compared to patients without oxaliplatin chemotherapy. Multivariate linear regression analysis demonstrated that anxiety and cognitive impairment performance significantly predicted for global Quality of Life (QOL). Conclusions: colorectal cancer (CRC)patients with oxaliplatin chemotherapy experience mood disorders, cognitive impairment, and reduced Quality of Life (QOL). The clinical symptoms severity of oxaliplatin cemotherapy plays an important role in mood change and cognitive function. Decreased Quality of Life (QOL) was associated with anxiety and cognitive impairment.
Keywords: Chemotherapy-induced neuropathy, oxaliplatin, colon and rectum carcinoma, psychological disorders, cognitive impairment, quality of life.
Introduction
Cancer is a lethal menace to human health while chemotherapy is one of the leading effective treatments (1). Colorectal cancer accounts for 10% to 15% and ranks top 2 in leading causes of death for all cancers (2). Metastatic diseases arise in about 50% of patients with colorectal cancer. Palliative chemotherapy enables patients to lengthen survival time and improve QOL. The latest methods that target critical biological pathways have provided more treatment options and a substantial improvement in survival and progression-free survival (PFS) for metastatic colorectal cancer (mCRC) patients. Oxaliplatinis, a third-generation platinum drug, is extensively used in first line treatment for CRC. However, cancer treatment can be interrupted by painful symptoms caused by undesirable side effects of various chemotherapy drugs, including oxaliplatin (3).
Platinum- and taxanes-derived drugs (oxaliplatin, cisplatin, carboplatin and paclitaxel) are likely to cause chemotherapy-induced neuropathy, which is one of the most serious side effects. Among these side effects, chemotherapy-induced peripheral neuropathy (CIPN) is a debilitating and dose-dependent side effect that interferes with cancer therapy regimens, significantly induces functional abilities loss, and affects QOL. Moreover, CIPN can lead to lowering of the dose and discontinuation of assumption, ultimately affecting overall survival ratio (4-6).
Sensory neuropathy symptoms include pain, allodynia, loss of sensation, paresthesia, numbness, tingling, and gait disturbance (7). CIPN normally caused by Taxanes, vinca alkaloids, platinum derivatives, bortezomib, and thalidomide, which predominantly impair afferent sensory fibers with a symmetric, distal, length-dependent “glove and stocking” distribution. Unfortunately, there are few strategies to treat this kind of neuropathy, partially due to the complexity of its pathogenesis (8). Therefore, it has great significance to develop analgesic drugs with novel mechanisms.
Patients with CRC have worse social and role dysfunction (9, 10). There have been few reports about the psychological, cognitive, QOL impairment of CRC patients following oxaliplatin chemotherapy and oxaliplatin-induced neuropathic pain. The comparative of psychological disorders and QOL between oxaliplatin chemotherapy patients and those without oxaliplatin chemotherapy is not fully understood. There is no significant evidence that chemotherapy causes psychological disorders. Therefore, we assessed the psychological functioning of CRC patients with and without oxaliplatin chemotherapy. We measured emotional wellness using the Zung Self-Rating Anxiety Scale (SAS) and Zung Self-Rating Depression Scale (SDS), cognitive function using the Montreal Cognitive Assessment (MoCA), and QOL using the World Health Organization’s Quality of Life instrument, abbreviated version (WHOQOL-BREF).
Methods
This research was approved by the Ethics Committee of The Xuan Wu Hospital. Patients included inpatients and outpatients of Xuan Wu Hospital, Capital Medical University. Written informed consent was signed from all participants.
Participants
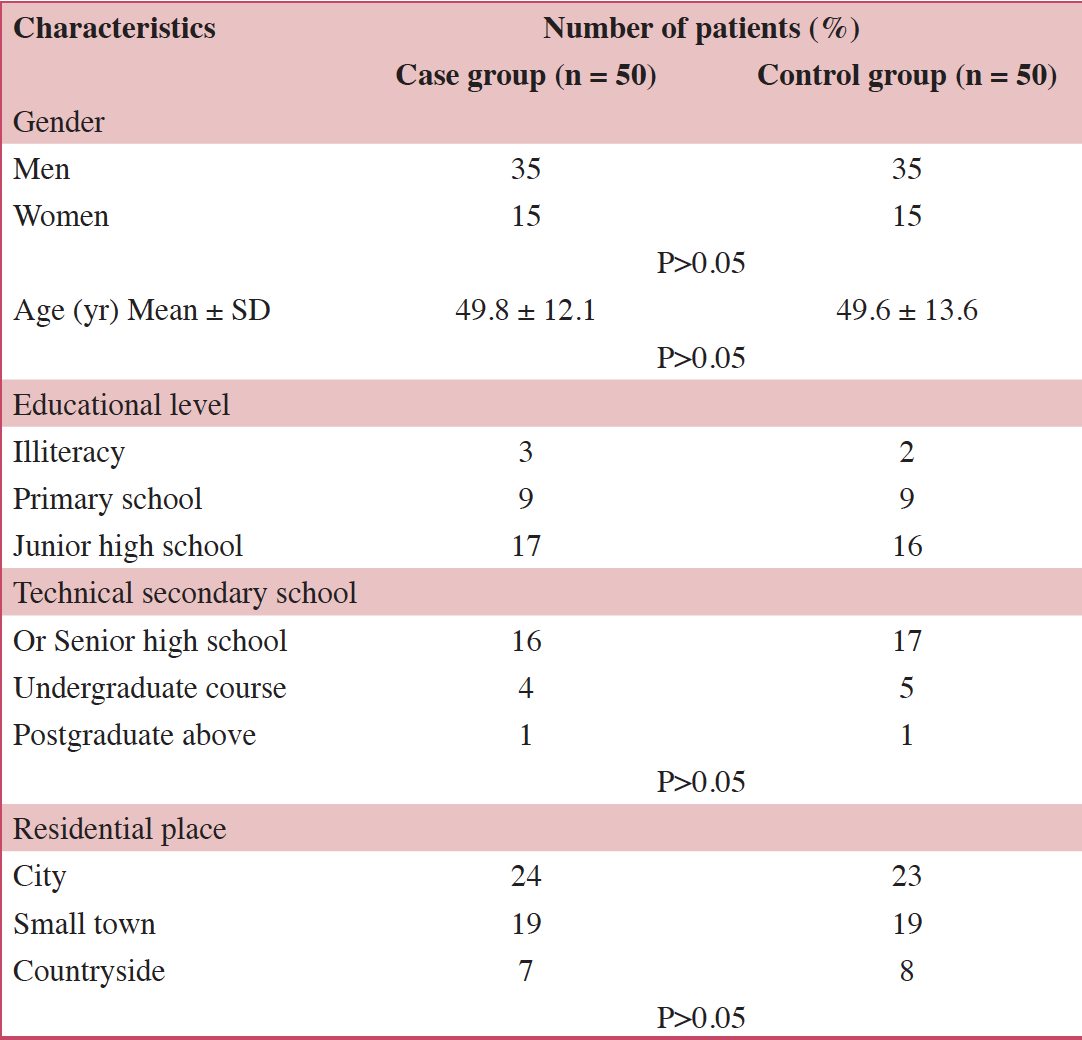
From September 2017 – December 2019, patients who fulfilled the following criteria were enrolled into the case group: (1) History of CRC with oxaliplatin chemotherapy; (2) no recurrent tumor, brain metastasis, brain tumor, brain abscess, cerebral infarction, encephalitis, demyelinating disease or other central nervous system (CNS) diseases; and (3) no consciousness disorder and vital sign changes. A total of 50 patients matched this criteria (35 males, 15 females) and were placed into the case group. During the same time period, [add control sample number] patients with a history of CRC and no history of oxaliplatin chemotherapy were recruited and placed into the control group. Control group participants met the following additional criteria. The age, gender, educational level, and treatment modalities of the subjects were matched. The same number of patients were recruited into the control group according to the inclusion criteria, which were the same as the case group, except for criteria (2).
Materials and Procedures
The following clinical information was collected from each patient: (1) age, gender, educational background, occupation and marriage status, residence area, and medical record (date of starting chemotherapy, dosage, the target volume, whether has another CNS diseases); (2) physical exam findings; and (3) oxaliplatin toxicity scores assessed by oxaliplatin neurotoxicity classification (for patients undergoing oxaliplatin chemotherapy).
Neuropsychological test
Self-Rating Depression Scale (SDS)
The Zung SDS is a 20-item self-report rating scale that measures symptoms of depression; cognitive condition, somatic symptoms, and psychomotor and emotional changes of the subjects were assessed via the scale. Each item is scored on a Likert scale ranging from 1 to 4. Participants with a total raw score of 25-43 do not exhibit clinical depressive symptoms, 50-59 are considered to have mild to moderate depression, etc. Participants with a total raw score of 54 or higher were labeled as having depression based on this scale (Chinese version).
Self-Rating Anxiety Scale (SAS)
The Zung SAS is a 20-item scale, with some of the items keyed positively and some negatively. They are answered on a four-point scale ranging from 1 (none or a little of the time) to 4 (most or all the time). After being converted into the standardized score, a cut-off 50 was used to define anxiety according to the scale (Chinese version).
Montreal Cognitive Assessment (MoCA)
The MoCA is a widely used screening tool for detecting early signs of dementia or mild cognitive decline. The MoCA assesses different cognitive domains: attention and concentration, executive function, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation. The time to administer the MoCA is approximately 10 minutes. The total possible score is 30. A score of 26 or above is considered normal.
WHOQOL-BREF
The WHOQOL-BREF is an abbreviated version of the WHOQOL-100, which measures the functional domains of an individual’s life deemed vital to a person’s quality of life. The WHOQOL-BREF is a 26-item measure that assesses an individual’s physical health (e.g., energy, mobility, sleep and rest), psychological health, social relationships, and environment (e.g., financial resources, home environment, physical safety).
Statistical analysis
SPSS for windows, version 13.0 was used for statistical analysis. Between the case group and control group, the clinical features and the SDS, SAS, MoCA, and WHOQOL-BREF scores were compared by a paired-sample t-test; the depression and anxiety scores were used for comparison by X2 test. Stepwise multiple linear regression was applied to explore predictors of psychological and cognitive disorders. Spearman’s correlation was performed to examine the relationship between oxaliplatin neurotoxicity classification and the scores of SDS, SAS, and MoCA. All tests were two-tailed, and the significance level was maintained at 5%.
Results
In total, 100 patients were recruited from Xuanwu Hospital. Fifty patients were placed into the case group and 50 were placed into the control group. The demographic data and baseline characteristic for the two groups were similar (Table 1). mFOLFOX and Xelox were applied to the primary tumor. Nineteen of the patients suffered from chronic underlying diseases such as hypertension, diabetes, chronic bronchitis and other medical co-morbidities.
Emotional Wellness
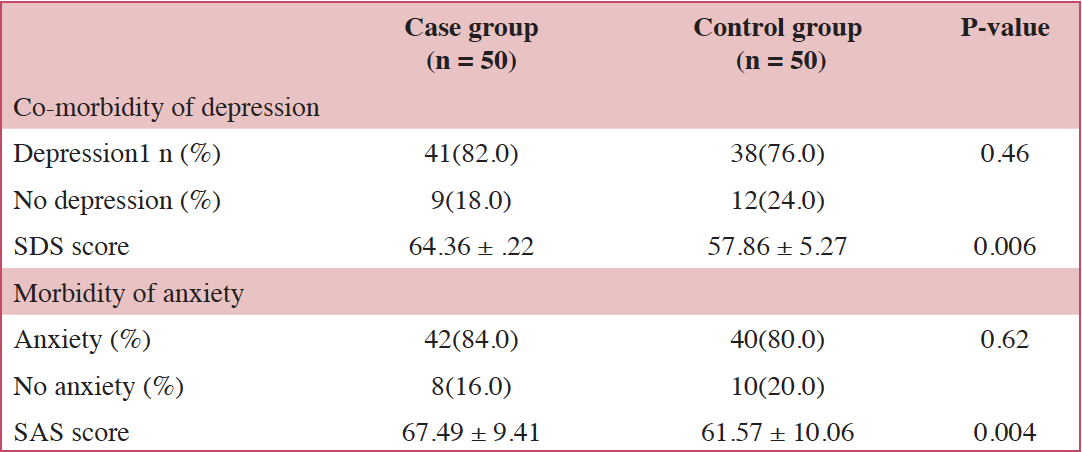
On measures of emotional wellness, 41 (82.0%) case group participants had depression, 42 (84.0%) had anxiety, and 38 (76.0%) had both. In the control group, 38 (76.0%) patients had depression, and 40 (80.0%) had anxiety. The differences in incidence rates of depression and anxiety between case group and control group participants were not statistically significant (SDS, 82% vs. 76%, p = 0.46; SAS, 84% vs. 80%, p = 0.62; Table 2); however, the standardized SDS score and SAS score were higher in the case group than those in the control group (64.36 ± 7.22 vs. 57.86 ± 5.27, P = 0.006; 67.49 ± 9.41 vs. 61.57 ± 10.06, P =0.004).
For the incidence of severe depression, the case group was 18% (nine patients) while the control group was 0% (no patient). The percentage of patients with severe depression, as defined by standardized SDS, was significantly higher in the case group compared with the control group (p= 0.018). In the case group, the number of patients with severe anxiety was 27 (54%). In the control group, the number of severe anxiety cases was 11 (22%); the percentage of patients with severe anxiety was significantly higher in the case group (p =0.005).
Cognitive function
The (mean) MoCA standardized scores from the case group and control group were (23.46 ± 3.17) and (27.49 ± 2.03), respectively. Patients without oxaliplatin chemotherapy tended to score higher than those with oxaliplatin chemotherapy (p<0.05).
Quality of Life
The raw scores are transformed into standard scores in line with the WHOOL-100 Instrument. The higher the score, the better QOL the patients felt. A comparison of QOL scores between the two groups are presented in Figure 1. Patients in the case group obtained a lower mean score (18.9 ± 7.8) on the domain of physical health of the WHOQOL-BREF compared to participants in the control group (37.8 ± 6.2, p<0.05). The mean score for psychological health of the cases was 19.3 ± 8.2, while the control score was 39.8 ± 8.1; data were considered significant in groups with P < 0.05. Additionally, regarding social relationships, the scores in the cases and the controls were 50.2 ± 10.1 and 70.6 ± 10.5 respectively; the difference was significant (p<0.05). In the environmental domain, the scores for the two groups were similar (49.6 ± 9.2 vs. 53.8 ± 9.3, p = 0.325, respectively).
To identify the determinants of QOL, the demographic data and scores of the SAS/SDS/MoCA were entered into the regression analysis. We found that the SAS score (p= 0.052) and MoCA score (p<0.005) were both the significant predictors (Table 3).
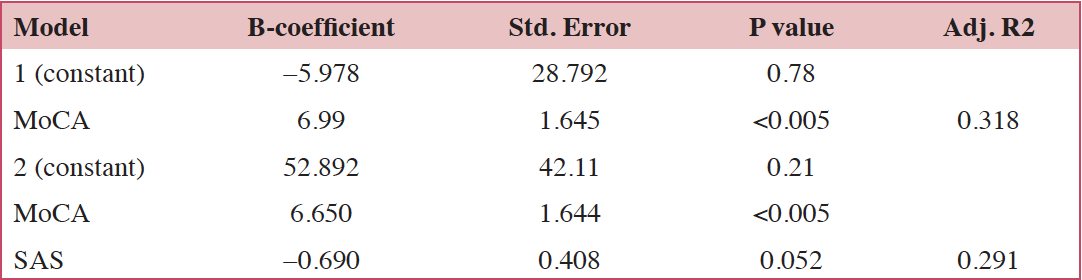
Table 3. Multiple linear regression analysis of age, gender, education, chemotherapy, SAS, SDS and MoCA to predict QOL
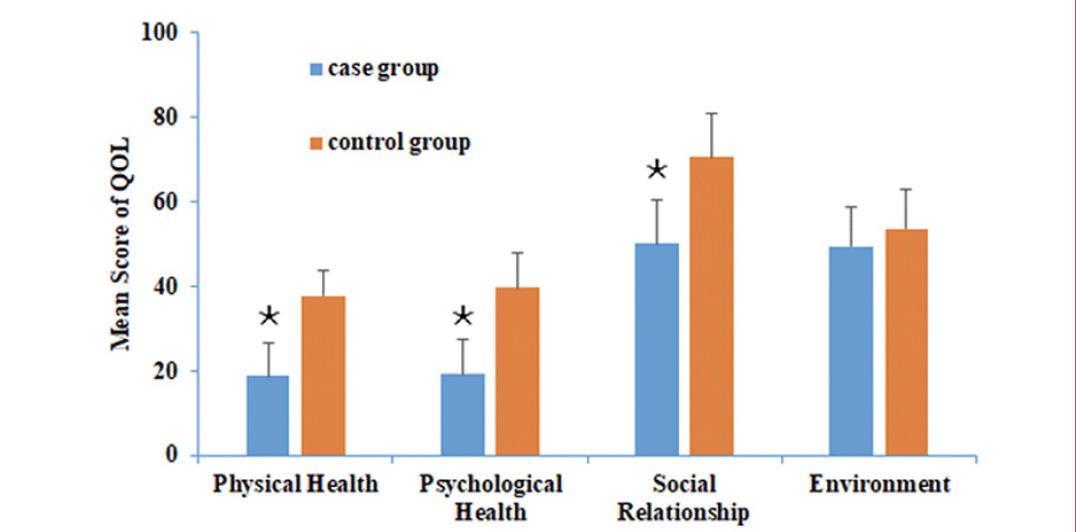
Figure 1. QOL in two groups. The X-axis represents the mean score per group on each of the four domains of the WHOQOL-BREF with a standard deviation of 6 represented by the vertical line
Participants in the case group obtained a lower score on the domains of physical health (p<0.05), psychological health (p<0.05), and social relationship (p<0.05). There was no statistically significant difference in mean scores on the environment domain between groups (p = 0.325). Abbreviations: QOL = quality of life
Discussion
This study evaluated emotional wellness (or mental health), cognitive function, and QOL of CRC patients with oxaliplatin chemotherapy. Data were compared with no-oxaliplatin chemotherapy CRC patients. More than three fourths of patients after oxaliplatin chemotherapy had either depression or anxiety based on SDS and SAS assessment. As reported previously, psychological disorders such as depression and anxiety were apparent as early as the start of chemotherapy and might even remain throughout the entire treatment (12-14). However, it is still not clear that how psychological disorders develop, and the mechanism of how they affect patients’ QOL during chemotherapy remains unresolved.
In this research, according to the SDS and SAS scores, depression and anxiety were more severe in patients with oxaliplatin chemotherapy than those without oxaliplatin chemotherapy. Regarding the factors influencing anxiety and depression, we found that age, gender, education, and chemotherapy had no significant correlation. Patients often are acutely aware of minute changes in their body during and following chemotherapy. Patients undergoing chemotherapy and those who have recently completed chemotherapy treatment often worry about the recurrence of tumors, and will frequently meet with their medical providers (citation), all of which are indicative of anxiety (citation).
MoCA can assess patients’ attention and concentration, executive function, memory, language, visuoconstruction abilities, conceptual thinking, calculations, and orientation. Ferlay J. founds that the late effects of chemotherapy on cognitive function include three situations: transitory cognitive impairment primarily affecting attention and recent memory; mild or moderate cognitive impairment and dementia with leukoencephalopathy occurring in the late delayed period (15). In our study, the case group participants/ participants with a history of chemotherapy performed worse on a cognitive function measure than patients without chemotherapy. Chemotherapy also proved to be a predictor of cognitive dysfunction.
In our study, the most common symptoms in patients with oxaliplatin chemotherapy included impaired cognition, bulbar palsy, headache, dizziness, and syncope. These symptoms significantly decreased patients’ QOL.
Presently, a cure for neurotoxicity to the nervous system, effective treatment strategies are missing. Chemotherapy-induced neuropathy is harmful toa patient’s QOL and leads to dose reduction or even treatment cessation. The current approaches to counteract the side effects of chemotherapy are not completely effective, fail to solve long-term consequences, and can induce other side effects (16-18).
Oxaliplatin (Third-generation platinum) differs from cisplatin due to the presence of an oxalate leaving group and a DACH (diaminocyclohexane) linker. Oxaliplatin is effective in cisplatin-resistant tumors because the DNA repair system does not recognize its adducts. Thus, it is widely used in colorectal cancer (19). Neves and Vargas pointed to epidemiological data demonstrating a large scale of use of platinum (monotherapy or in combination with other drugs) in clinical oncology (20). Patients with NPC after oxaliplatin chemotherapy often have chemotherapy-induced neuropathy. It predominantly impairs afferent sensory fibers with a symmetric, distal, length-dependent “glove and stocking” distribution. Our score of QOL showed a significant difference between patients with oxaliplatin chemotherapy and patients without oxaliplatin chemotherapy in the following domains: physical and psychological health, and social relationships. Regression analysis also found that anxiety and cognitive impairment might explain the lower QOL scores.
Funding Support
This work was supported by the Beijing Municipal Administration of Hospitals’ Youth Programme (QMS20180805); Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan (CIT&TCD201904093); Beijing Excellent Talents Training Funding Project (2018000026833ZK78); and the Xuanwu Hospital Huizhi Talent Project (XW2019091680124).
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance and scientific consultation during the preparation of this manuscript.
Conflicts of Interest
The authors have declared that no competing interests exist.
Ethical standards
This research was approved by the Ethics Committee of The Xuan Wu Hospital. All participants gave informed consents.
References
1. Cavaletti G., and Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015; 28, 500-507.
2. Chamberlain, M.C. Neurotoxicity of cancer treatment. Curr. Oncol. Rep. 2010; 12, 60-67.
3. Han, Y.; Smith, M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013; 4, 156-163.
4. Ben Abdallah, N.M., et al. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging. 2010; 31 (1): 151-161.
5. Areti, A., Yerra, V. G., Naidu, V., et al. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014; 2, 289-295.
6. Barton, D. L., Wos, E. J., Qin, R., et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support. Care Cancer. 2011; 19, 833-841.
7. Brown, T. J., Sedhom, R., and Gupta, A. Chemotherapy-induced peripheral neuropathy. JAMA Oncol. 2019; 5:750-768.
8. Shin HR, Masuyer E, Ferlay J, et al. Cancer in Asia – Incidence rates based on data in cancer incidence in five continents IX (1998–2002). Asian Pac J Cancer Prev. 2010; 11 Suppl 2: 11-16.
9. Heinonen H, Aro AR, Aalto AM,et al. is the evaluation of the global quality of life determined by emotional status? Qual Life Res.2004; 13: 1347-1356.
10. Grisold W., Cavaletti G., and Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-oncology. 2012; 14, 45-54.
11. Baranowski A., Abrams P., Berger R., et al.d. C. Classification of Chronic Pain, 2nd ed. revised, IASP Task Force on Taxonomy, Seattle, WA. 2018; 12-21.
12. Umana, I.C., Daniele, C.A., Miller, B.A., et al.Nicotinic modulation of descending pain control circuitry. Pain. 2017; 158, 1938-1950.
13. Umana, I.C., Daniele, C.A., Miller, B.A., et al. Nicotinic modulation of descending pain control circuitry. Pain. 2017; 158, 1938-1950.
14. Pacini, A., Micheli, L., Maresca, M., et al. The alpha9alpha10 nicotinic receptor antagonist alpha-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol. 2016; 282, 37-48.
15. Ferlay, J., Soerjomataram, I., Dikshit, R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015; 136, E359-E386.
16. Ferré S., Casadó V., Devi LA., et al. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol. Rev. 2014; 66, 413-434.
17. Hou, S., Huh, B., Kim, H. K., et al. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician. 2018; 21, 571-592.
18. Ye, H., Du, X., Hua, Q., Effects of voluntary exercise on antiretroviral therapy-induced Neuropathic pain in mice. J. Physiol. Sci. 2018; 68 (4), 521-530.
19. Toma, W., et al. Effects of paclitaxel on the development of neuropathy and affective Behaviors in the mouse. Neuropharmacology. 2017; 117, 305-315.
20. Trecarichi, A., and Flatters, S. J. L. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int. Rev. Neurobiol. 2019; 145, 83-126