*Suggested Citation: Fernandes-Alcantara, A. L. (2017). Vulnerable youth: Employment and job training programs (CRS Report R40929). Washington, D.C.: Congressional Research Service.
Published online November 9, 2017, http://dx.doi.org/10.14283/cw.2017.10
Summary
In an increasingly global economy, and with retirement underway for the Baby Boomer generation, Congress has indicated a strong interest in ensuring that today’s young people have the educational attainment and employment experience needed to become highly skilled workers, contributing taxpayers, and successful participants in civic life. Challenges in the economy and among certain youth populations, however, have heightened concern among policymakers that some young people may not be prepared to fill these roles. The employment levels for youth under age 25 have declined markedly in recent years, including in the wake of the 2007-2009 recession. Certain young people—such as high school dropouts, current and former foster youth, and other at-risk populations—face challenges in completing school and entering the workforce.
Since the 1930s, federal job training and employment programs and policies have sought to connect vulnerable youth to work and school. Generally, these young people have been defined as being at-risk because they are economically disadvantaged and have a barrier to employment.
During the Great Depression, the focus was on employing young men who were idle through public works and other projects. The employment programs from this era included an educational component to encourage youth to obtain their high school diplomas. Beginning in the 1960s, the federal government started funding programs for low-income youth that address their multiple needs through job training, educational services, and supportive services.
Currently, there are four major federal youth employment and job training programs, all of which are administered by the Department of Labor’s (DOL’s) Employment and Training Administration (ETA). Although these programs have varying eligibility requirements and are carried out under different funding arrangements, they generally have a common purpose—to provide vulnerable youth with educational and employment opportunities and access to leadership development and community service activities. The Youth Activities program offers job training and other services through what are known as local workforce development boards, whose members are appointed by the chief local elected official(s). The program was funded at $873.4 million in FY2016. The Job Corps program provides career and technical training in a number of trades at 125 residential centers throughout the country. The program received FY2016 appropriations of $1.7 billion. Another program, YouthBuild, engages youth in educational services and job training that focus on the construction trades. YouthBuild received FY2016 appropriations of $84.5 million. Separately, the Reentry Employment Opportunities program, formerly known as the Reintegration of Ex-Offenders program, includes job training and other services for juvenile and adult offenders. The youth component of the program was funded at $39.5 million in FY2016.
The four programs were authorized under the Workforce Investment Act of 1998 (WIA, P.L. 105-220) through FY2003, and Congress continued to appropriate funding for the programs in subsequent years. On July 22, 2014, President Obama signed into law the Workforce Innovation and Opportunity Act (WIOA, P.L. 113-128). WIOA amended these programs, particularly the Youth Activities program and Job Corps. Like WIA, WIOA does not explicitly authorize the Reentry Employment Opportunities program; however, Congress appropriated funding for the program in FY2016 (P.L. 114-113) under the authority of Section 169 of WIOA and the Second Chance Act. Section 169 authorizes evaluations and research. The amendments made by WIOA generally went into effect on July 1, 2015.
Introduction
In an increasingly competitive economy, and with retirement underway for the Baby Boomer generation, Congress has indicated a strong interest in ensuring that today’s young people have the educational attainment and employment experience necessary to become highly skilled workers, contributing taxpayers, and successful participants in civic life. Challenges in the economy and among vulnerable youth populations, however, have heightened concern among policymakers that many young people may not be prepared to fill these roles.
The employment levels for youth under age 25 have generally declined since 2000, though attachment to the workforce has improved for this population in the wake of the recession that extended from December 2007 through June 2009 (1). Certain young people in particular— including those from low-income families, high school dropouts, foster youth, and other at-risk populations—face barriers to completing school and entering the workforce. Since the 1960s, federal job training programs and policies have sought to connect these youth to education and employment pathways. Contemporary federal employment programs with this same purpose include the Youth Activities program; Job Corps; YouthBuild; and the Reentry Employment Opportunities (REO) program, which includes a youth component. These programs provide a range of services and supports to youth. Some of the programs concentrate on specific job trades and/or serve targeted at-risk populations. The programs were previously authorized under the Workforce Investment Act (WIA) of 1998 (P.L. 105-220). On July 22, 2014, President Obama signed into law the Workforce Innovation and Opportunity Act (WIOA, P.L. 113-128). WIOA superseded WIA and made significant amendments to the youth programs (2). Changes made by WIOA generally went into effect on July 1, 2015 (3).
This report provides an overview of federal employment programs for vulnerable young people. It begins with a discussion of the current challenges in preparing all youth today for the workforce. The report then provides a chronology of job training and employment programs for at-risk youth that began in the 1930s and were expanded or modified from the 1960s through the 1990s. It goes on to discuss the four youth programs authorized under WIOA, and draws comparisons between these programs. Following this section is a detailed discussion of each of the programs.
Context
As they leave high school, either through graduation or by dropping out, young people can pursue various options. Youth with a high school diploma may attend a two- or four-year college, enlist in the armed services, or secure part-time or full-time employment (sometimes paired with attending school). Youth without a high school diploma can do some of these same things, but their opportunities are more limited. They cannot enroll in a four-year college or, in most cases, enlist in the military. These youth will likely have difficulty supporting themselves if they do work (4).
In fact, individuals who drop out are less likely to secure employment and have lower earning power. As the level of education rises, the unemployment rate decreases and median weekly earnings increase for those who work (5). In 2015, among workers with less than a high school degree, the unemployment rate was 8.0% and earnings averaged $493 per week. This is compared to an unemployment rate of 5.4% and $678 in weekly earnings for workers with a high school degree. Workers with a bachelor’s degree had an unemployment rate of 2.8% and median weekly earnings of $1,137. With the shift to a knowledge-based economy, many new jobs will require some college education or better. According to DOL’s Bureau of Labor Statistics (BLS), the fastest growing occupations between 2014 and 2024 will require some postsecondary education (6). The costs of dropping out extend beyond the individual’s foregone job opportunities and lower wages. According to the research literature, costs can be incurred by society overall. These costs include possible lost payroll tax revenue and increased transfers for welfare payments, imprisonment, and programs to re-enroll dropouts in school (7).
Federal youth employment and job training programs have long targeted services to young people who leave school before graduating or are in school and may be vulnerable to dropping out. The purpose of these programs, as they currently exist, is to provide job training, employment, educational services, and social services that can help youth become economically self-sufficient and achieve their career and academic goals. These contemporary programs also emphasize leadership development and community service. Note that while youth employment and job training programs are also enhanced with state workforce and other dollars, the extent to which this support is provided is unclear.
History of Federal Youth Employment and Job
Training Programs (8)
For more than 90 years, the federal government has played a role in helping young people secure employment and achieve academic success. Generally, these young people have been defined as being vulnerable in some way—either because they are economically disadvantaged and/or have a barrier to securing employment or completing their education. During the Great Depression, the focus was on employing idle young men in public works and other projects. The employment programs from this era included an educational component to encourage youth to obtain their high school diplomas. Beginning in the 1960s, the federal government started funding programs for low-income youth, such as Job Corps, that address their multiple needs, including job training, educational services, housing, and supportive services. During the 1970s and 1980s, Job Corps was expanded and the federal government funded additional programs for both in-school and out- of-school youth. Funding was also appropriated to test the efficacy of some of these programs.
The Workforce Investment Act of 1998 extended earlier programs and created new ones, with the intention of providing more seamless job training and education services for youth year-round.
The Workforce Innovation and Opportunity Act maintained these programs and changed some of their requirements. Generally, these programs are targeted to teenagers and young adults, usually to age 24, who are at risk of dropping out or have already done so.
Depression Era
Prior to the 1930s, the federal government’s involvement in youth employment was primarily limited to regulating child labor (9). The Great Depression served as a catalyst for the creation of federal programs to employ and educate young people who were out of work or at risk of dropping out of school due to financial difficulties. The Civilian Conservation Corps (CCC) began in 1933 as an employment program for unemployed males ages 18 to 25 (and veterans, Indians, and residents of territories of any age) to participate in projects planned by the Departments of the Interior and Agriculture. These projects focused on creating and improving infrastructure, transportation, and recreational services, among other categories. The young men lived in camps and were provided with an allowance, food, and medical care. The CCC also included an educational component, which taught nearly 35,000 participants to read and write and assisted a smaller number with attaining their high school and college degrees. Until the program ended in 1945, it served nearly 3 million men, of whom approximately 10% were veterans.
Other Depression era programs—the Student Aid program, Works Project program, and Guidance and Placement program—were administered by the National Youth Administration, which was created as part of the now-defunct Works Progress Administration by an executive order in 1935. The programs provided funds for part-time employment of needy high school, college, and graduate students to assist them in completing school, as well as funds for part-time employment for unemployed out-of-school youth. These young people, all of whom were ages 16 through 25, were employed in a number of broad areas, including construction, clerical work, and research.
These programs served hundreds of thousands of youth before they were discontinued in the early 1940s.
War on Poverty Programs
The 1960s marked a period of federal efforts to assist poor and disadvantaged children, adolescents, and their families through job training and other programs. In response to concerns about high unemployment, the Manpower Development and Training Act of 1962 (P.L. 87-415) and subsequent amendments to it authorized funding for employment training. Specifically, amendments to the act in 1963 (P.L. 88-214) encouraged the Department of Labor to provide assistance to youth so that they might be able to successfully enter the labor force, and expanded the share of job training funds that could be used to train youth under age 22 from 5% to 25%.
Further, federal funding was first authorized through the 1963 amendments to provide employment opportunities to youth from low-income families.
President Lyndon B. Johnson’s subsequent War on Poverty established new youth-targeted programs in job training and educational assistance under an initiative known as the Neighborhood Youth Corps (NYC). The NYC was made up of work training programs, the Work Study program, and Job Corps. The work training programs provided work experience, job training, and supportive services to low-income unemployed youth ages 16 through 21 who were in school or out of school, including dropouts. The Work Study program was modeled on the Depression-era Student Aid program and provided money to high school and college students from low-income families who needed earnings to stay in school. The program continues today for college students. Job Corps, which also continues today, was established under the Economic Opportunity Act of 1964 (P.L. 88-452) to provide educational and job training opportunities to disadvantaged youth at residential and non-residential centers. (See “Job Corps,” below, for further information.)
Expanding Youth Programs
The 1973 Comprehensive Employment and Training Act (CETA, P.L. 93-203) was the first of four laws enacted during the 1970s and 1980s that focused greater federal attention on youth employment and training. The second law, the Youth Employment and Demonstrations Project Act (YEDPA, P.L. 95-93) was enacted in 1977 and established a variety of employment, training, and demonstration programs for youth. The 1982 Job Training Partnership Act (JTPA, P.L. 97-300 repealed CETA. JTPA was subsequently repealed by WIA. Separately, the School-to-Work Opportunities Act of 1994 (STWOA, P.L. 103-239) supported the development of programs that encouraged students to pursue learning opportunities and experiences that incorporated occupational skills. Activities authorized under these acts were administered by DOL. STWOA was additionally carried out by the Department of Education (ED).
Comprehensive Employment and Training Act (CETA) and Youth Employment and Demonstrations Project Act (YEDPA)
As amended through 1978, CETA authorized a range of employment and training programs for adults and youth. Job Corps and the Summer Program for Economically Disadvantaged Youth (SPEDY) were the primary youth programs authorized under CETA. SPEDY provided funding to employers to hire low-income youth ages 14 through 21 during the summer months. Youth served as assistants in hospitals, libraries, community service organizations, and schools, among other settings.
The Youth Employment and Demonstrations Project Act (YEDPA), signed into law in 1977, amended CETA (10). YEDPA increased authorization of appropriations for Job Corps and SPEDY and authorized three additional programs targeted to “economically disadvantaged” (defined under the act) youth ages 14 through 21: Youth Employment and Training Programs (YETP), Youth Community Conservation and Improvement Projects (YCCIP), and Youth Incentive Entitlement Pilot Projects (YIEPP) (11). YEDPA was passed in response to high levels of unemployment among youth relative to adults, even during periods of economic expansion, and growing gaps in youth unemployment among whites and blacks, males and females, and in- school and out-of-school youth. The programs were carried out during the Carter Administration, from 1977 through 1981. Over this period, YEDPA served 6.1 million youth.
YETP and YCCIP were intended to meet the immediate employment needs of youth, and funding for the programs was allocated primarily on a formula basis. YETP activities include work experience, pre-employment skills, and an emphasis on the transition from school to work.
YCCIP was intended to assist unemployed, out-of-school youth obtain a high school degree, conditional on satisfactory performance in work and school. Further, it was aimed at improving coordination between the job training and educational systems as a means of addressing the dropout problem (12). Finally, YIEPP funded evaluations to test the efficacy of demonstration programs; the other two programs included funding for demonstration programs. During the YEDPA years, more than 60 major demonstrations were funded in about 300 sites, operated by DOL in cooperation with six other federal agencies and private nonprofit intermediaries.
Job Training Partnership Act (JTPA)
CETA was repealed in 1982 by the Job Training Partnership Act (13). JTPA was distinct from its predecessor because it emphasized that states and localities, rather than the federal government, had the primary responsibility for administering job training and employment programs. Funding was appropriated under JTPA through FY1999. JTPA programs focused on the training needs of “economically disadvantaged” (defined under the act) youth and adults facing significant barriers to employment. JTPA programs included the Summer Youth Employment and Training program, the Youth Training Program, and Job Corps.
The Summer Youth Employment and Training program provided employment and training activities during the summer months for low-income youth ages 14 through 21 to strengthen basic educational skills, encourage school completion, provide work exposure, and enhance citizenship skills. In the summer of 1997, an estimated 500,000 youth participated. The Youth Training Program was established by the Job Training Reform Amendments of 1992 (P.L. 102-367), which amended JTPA to address concerns that school dropouts were not being reached by the then- existing combined program for disadvantaged adults and youth, and that the program primarily served youth who were the easiest to place in jobs and required the fewest services (14). The program was year-round and provided direct services, such as on-the-job training, tutoring and study skills training, and school-to-work transition services. It also provided training-related and supportive services, including job search assistance, drug and alcohol abuse counseling, and cash incentives based on attendance and performance in a program. Economically disadvantaged in- school and out-of-school youth ages 16 through 21 were eligible, but 50% of participants had to be out of school. Further, at least 65% of youth had to be hard to serve, meaning they were school dropouts (if out of school), pregnant or parenting, or offenders, among other qualifications. In program year 1997, an estimated 107,000 youth participated. JTPA was replaced by WIA.
School to Work Opportunity Act (STWOA)
The School to Work Opportunity Act of 1994 authorized the School-to-Work (STW) program administered jointly by DOL and the Department of Education through the National School-to- Work Office (15). The program was funded from FY1994 through FY2000. The law supported the development of programs with three main elements: work-based learning to provide participating students with work experience and on-the-job training; school-based learning, involving upgrading and integrating the occupational skills participating students learn in school and the workplace; and program coordination to aid the planning, implementation, and operation of the program. STWOA grants were competitively awarded to states, local partnerships, programs for Indian youth, and U.S. territories to implement school-to-work systems. In addition, STWOA authorized national activities, such as research and demonstrations. Some school-to-work programs that received seed money from the federal program continue to operate today.
Workforce Investment Act (WIA)
The Workforce Investment Act of 1998 replaced JTPA. WIA includes titles that authorize programs for job training and related services (Title I), adult education and literacy (Title II), employment services (Title III), and vocational rehabilitation (Title IV). Title I of WIA authorized job training programs for youth, adults, and dislocated workers (16). Funding was authorized for the program through FY2003, and Congress continued to appropriate funding for the programs in subsequent years.
Workforce Innovation and Opportunity Act (WIOA)
Congress took steps toward reauthorizing WIA from the 108th to the 113th Congresses, ultimately passing the Workforce Innovation and Opportunity Act (WIOA) on July 9, 2014. President Obama signed the bill into law (P.L. 113-128) on July 22, 2014. As of July 1, 2015, the law superseded WIA. Like WIA, WIOA includes titles that authorize programs for job training and related services (Title I), adult education and literacy (Title II), employment services (Title III), and vocational rehabilitation (Title IV). The major job training programs for youth and other workers are authorized in Title I.
Overview of Youth Programs Authorized Under Title I of WIOA
WIOA authorizes, and Congress has funded, three job training and employment services for youth:
• Youth Workforce Investment Activities Program (hereinafter, Youth Activities Program), a formula grant program for state and local workforce development boards (WDBs) that includes employment and other services that are provided year-round;
• Job Corps, a program that provides job training and related services primarily at residential centers maintained by contractor organizations; and
• YouthBuild, a competitive grant program that emphasizes job training and education in the construction trades.
As mentioned, Job Corps was enacted as part of the Economic Opportunity Act of 1964 (P.L. 88- 452), and was later incorporated into CETA, JTPA, and WIA. YouthBuild was originally authorized under the Cranston-Gonzalez National Affordable Housing Act of 1992 (P.L. 102- 550). The program was administered by the Department of Housing and Urban Development (HUD) until it was transferred to DOL in 2007 under the YouthBuild Transfer Act (P.L. 109-281) and incorporated into WIA. Under WIA’s pilot and demonstration authority, DOL established the Reintegration of Ex-Offenders (ReXO) program, a program for juvenile and adult offenders that provides job training and other services. WIOA does not explicitly authorize the program, now known as the Reentry Employment Opportunities (REO) program; however, Congress appropriated funding in FY2016 (P.L. 114-113) under the authority of Section 169 of WIOA and the Second Chance Act. Section 169 authorizes evaluations and research.
DOL’s Employment and Training Administration (ETA) administers the four programs. All of the programs offer employment, job training, and educational services. For example, local workforce development areas must provide specific elements, including mentoring and follow-up, to youth who receive services under the Youth Activities program. YouthBuild program participants engage in employment and other activities primarily related to housing and other types of construction work. Job Corps is the only one of the programs that provides residential services; youth can live onsite and receive health care services, child care, and other supports. Further, the programs generally serve vulnerable youth. Participants in YouthBuild and Job Corps must be low-income and have specified barriers to employment. The same is true in the Youth Activities program, except those who are out-of-school do not have to be low-income. The youth component of the Reentry Employment Opportunities program serves youth who have become involved in the juvenile justice or criminal justice system or youth at risk of becoming involved. The programs are funded somewhat differently. DOL allocates funding for the Youth Activities program to state WDBs based on a formula, while Job Corps enters into contracts with nonprofit and for-profit organizations and into an interagency agreement with the U.S. Department of Agriculture’s Forest Service. The other programs competitively award grants to nonprofit and other organizations and local communities. Table 1 summarizes the programs’ major features.
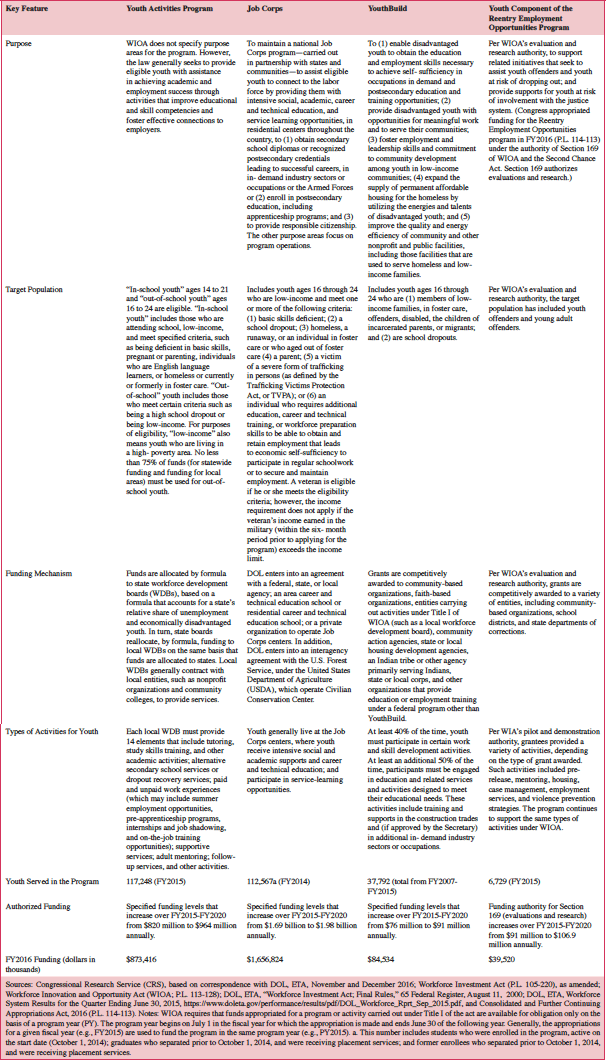
Table 1. Features of Youth Programs As Authorized Under Both WIA and (as of July 1, 2015) WIOA
Coordination
The WIOA Youth program and other youth programs make up a network of job training and employment opportunities for youth. In some communities, this may be formalized while in others, coordination between the programs may be less structured. WIOA includes provisions that encourage or require the programs to coordinate with one another. The state workforce board may include representatives of organizations that have demonstrated experience and expertise in addressing the employment, training, or education needs of eligible youth, including representatives of organizations that serve out-of-school youth (17). These boards are responsible for carrying out WIOA programs at the state level and allocating funds to local WDBs.
Further, under the state workforce plan (“unified state plan”), states are required to submit a description of the state’s strategic vision and goals for preparing an educated and skilled workforce—including preparing youth and individuals with barriers to employment—and for meeting the skilled workforce needs of employers, among other requirements (18). In addition, local WDBs, which receive funds to carry out the Youth Activities program are required, as part of their local plans, to describe and assess the type and availability of youth workforce investment activities in the local area, including activities for youth with disabilities. The plan must identify successful models of such youth workforce investment activities (19). Local workforce boards may choose to establish a standing committee to provide information and assist with planning to provide services to youth (20). Further, the Youth Activities program, Job Corps, and YouthBuild are required partners at one-stop centers. One-stop centers are operated by local WDBs and include federal programs that coordinate employment and other services in a community for all youth and adults (21).
Funding
WIOA provides funding authorization from FY2015 through FY2019 for youth employment and job training programs. Funds appropriated for a program or activity carried out under Title I of the act are available for obligation on the basis of a program year (22). The program year begins on July 1 in the fiscal year for which the appropriation is made and ends June 30 of the following year. Funds for the Youth Activities program may first become available for a new program year in the preceding April. In addition, Congress has tended to specify that funds appropriated for YouthBuild and the youth component of the Reentry Employment Opportunities program are available for obligation beginning in the April preceding a given program year. Congress has generally required that obligated funds for Job Corps are made available for one program year, although funding for certain purposes can be obligated through later dates. Funds obligated for any program year for the Youth Activities may be expended by each state receiving such funds during that program year and the two succeeding program years. Local areas may expend funds received from the state during the program year and the succeeding program year (23).
Funding for FY2000-FY2016
Table 2 includes the level of funds appropriated to each of the youth job training and employment programs for FY2000 through FY2016. Appropriations for these years correspond to the same program year, and are reported as such in the table (i.e., PY2000 through PY2016). Congress appropriated a total of $2.4 billion to $2.8 billion annually for these programs in most years over this period. Table A-1 in the Appendix presents Youth program funding allocated to the states and outlying areas for PY2009 through PY2016.
Job Corps has generally received the largest appropriation each year, followed by the Youth program, YouthBuild, and the youth component of the Reintegration of Ex-Offenders (although in two years, YouthBuild received less funding than the ReXO youth component).
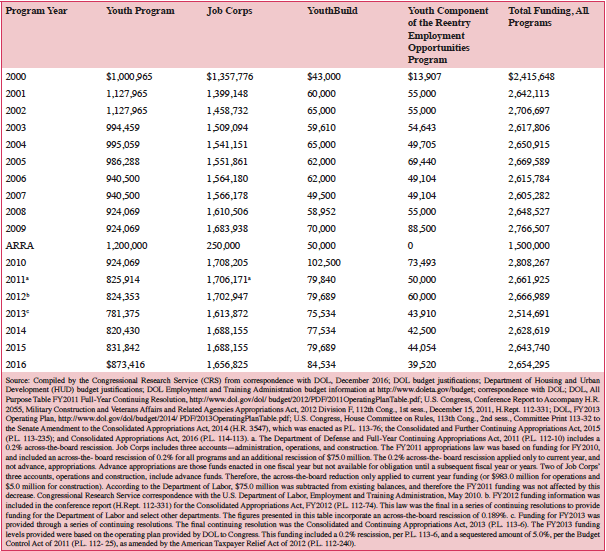
Table 2. Appropriations for DOL Youth Job Training and Employment Programs, PY2000-PY2016 and Under the American Recovery and Reinvestment Act (ARRA, P.L. 111-5)
Dollars in thousands; the fiscal year generally corresponds to the program year for each program
FY2016 Funding
Following three continuing resolutions (P.L. 114-53, P.L. 114-96, and P.L. 114-100), Congress passed, and President Obama enacted, the Consolidated Appropriations Act, 2016 (P.L. 114-113) to fund the Department of Labor and other agencies. FY2016 funding for the four youth job training and employment programs totaled $2.7 billion. Funding increased from FY2015 by nearly $42 million for the Youth program and nearly $5 million for the YouthBuild program. The funding for the youth component of the Reentry Employment Opportunities program decreased by $4.5 million and for Job Corps by $3.1 million.
FY2015 Funding
Following two continuing resolutions (P.L. 113-164 and P.L. 113-202), Congress passed, and President Obama enacted, the Consolidated and Further Continuing Appropriations Act, 2015 (P.L. 113-235) to fund DOL programs through FY2015. Funding for the youth programs totaled $2.6 billion. Funding increased from FY2014 by over $11 million for the Youth program; over $7 million for the YouthBuild program; and nearly $2 million for the youth component of the Reentry Employment Opportunities program; Job Corps funding remained level.
FY2014 Funding
FY2014 (PY2014) appropriations were not enacted prior to the beginning of the fiscal year (October 1), resulting in a 16-day shutdown of the federal government. On October 16, 2013, the Senate and House agreed to a bill (H.R. 2775) to provide temporary government-wide FY2014 funding through January 15, 2014 (or until full-year funding was appropriated). This bill was signed by the President on October 17, 2013 (P.L. 113-46). A second short-term continuing resolution (P.L. 113-73) extended appropriations through January 18, 2014. On January 17, 2014, the President signed into law the Consolidated Appropriations Act, 2014 (P.L. 113-76) to fund appropriations through September 30, 2014. In total, $2.6 billion was appropriated for youth job training and employment programs.
The next section of the report provides further discussion about the youth programs authorized under Title I of WIOA.
WIOA Youth Activities Program (24)
Overview and Purpose
The Youth Activities program is one of three formula grant programs that were initially authorized by WIA, and is now authorized under WIOA as the Youth Workforce Investment Activities program. The other two WIOA programs target adults (Adult Activities) and dislocated workers (Dislocated Worker Activities), although youth ages 18 or older are eligible for services provided through the Adult Activities program. These programs provide core funding for a coordinated system of employment and training services overseen by a state workforce development board and the governor, and composed of representatives of businesses and other partners. WIOA does not include purpose areas for the Youth Activities program; however, it generally seeks to provide assistance to youth in achieving academic and employment success.
Program Structure
With assistance from the state workforce development board, the governor develops a plan (known as the unified state plan) that is submitted to DOL. The plan is to address several items related to employment and training needs, performance accountability, and employment and training activities. The plan is to be submitted every four years for the Youth Activities, Adult Activities, and Dislocated Activities programs (25). Further, the unified state plan is to address youth primarily in two places. It must outline the state’s strategic vision and goals for preparing an educated and skilled workforce, include preparing youth with barriers to employment. It must also outline the criteria to be used by local boards in awarding contracts for youth services and describing how local WDBs will take into consideration the ability of providers to meet performance measures that are based on primary indicators of performance for the Youth Activities program (these indicators are discussed in a subsequent section).
As specified under WIOA, a local workforce area is overseen by the local workforce development board. The local board is made up of partners that collaborate to provide coordinated employment and training services in the community (26). Membership of the local board is to include representatives of businesses, local education entities, labor organizations, community-based organizations, and economic development agencies, among others (27). The boards may include representatives of organizations that have demonstrated experience and expertise in addressing the employment, training, or education needs of eligible youth, including representatives of organizations that serve out-of-school youth (28).
Local boards are to competitively award funds to local organizations and other entities to provide employment and job training services to youth (29). Grants or contracts awarded are to be based on criteria in the state plan, and by taking into consideration the ability of the providers to meet performance accountability measures that are based on primary indicators of performance for the Youth Activities program. Further, a local board may award funding on a sole-source basis if the board determines there is an insufficient number of eligible providers of youth workforce investment activities in the local area to participate on a competitive basis. Local boards may terminate “for cause” the eligibility of these providers (30).
The local board develops a local plan that discusses items similar to those in the state plan, except that the plan describes the local area’s one-stop delivery system. A one-stop system is intended to provide central access to employment and training services in a community. The Youth Activities program is a required partner in the one-stop system under WIOA. The WIOA regulations specify that local boards must either collocate youth program staff at one-stop centers and/or ensure one- stop centers and staff are equipped to advise youth in order to increase youth access to services and connect youth to the program that best aligns with their needs (31). Further, one-stop systems may have specialized centers to address special needs. WIOA specifies that this may include the needs of youth.
The local board must ensure that parents and other stakeholders are involved in designing and implementing the Youth Activities program (32). In addition, the local board may establish a standing committee to provide information and to assist with planning, operational, and other issues relating to providing services to youth, including community-based organizations with a demonstrated record of success in serving eligible youth (33). If a local board establishes a standing youth committee, it may assign it the responsibility of selecting youth providers. The WIOA regulations discuss the potential role of a standing youth committee, including to recommend policy direction to the local board for the design and development of programs to benefit all youth; the design of a comprehensive community workforce development system to ensure a full range of services and responsibilities for all youth, including disconnected youth; and ways to leverage resources and coordinate services among schools, public programs, and community- based organizations serving youth, among other possible responsibilities (34).
Allocations
Funding for the Youth Activities program is allocated from DOL to states, including Puerto Rico and Washington, DC, and the outlying areas (35). WIOA requires that not more than 0.25% is reserved for outlying areas and not more than 1.5% is reserved for youth activities in programs to serve Native American youth (36). WIOA specifies that the allotments for the outlying areas are based on a competitive grant process (37).
The remainder of the funds are allocated to states by a formula. The formula is based (1) one- third on the relative number of unemployed individuals residing in areas of substantial unemployment (an average unemployment rate of at least 6.5% for the most recent 12 months); (2) one-third on the relative “excess” number of unemployed individuals (an unemployment rate of at least 4.5%); and (3) one-third on the relative number of disadvantaged youth (individuals 16 through 21 who receive an income that, in relation to family size, does not exceed the higher of the poverty line or 70% of the lower living standard income level) (38). WIOA specifies that states are to receive, at minimum, the higher of 90% of their relative share of the prior year’s funding or, at maximum, 130% of their relative share of the prior year’s funding (39).
Of the funds allocated to states for the Youth Activities program (as well as for the Adult and Dislocated Worker programs), not more than 15% can be reserved for statewide activities (only 5% of reserved funds may be used for administrative activities, per WIOA) (40). States must use these funds for certain specified activities, and may use the funds for other specified activities. For example, WIOA requires states to use the statewide funds to carry out monitoring and oversight activities of the Youth Activities program (and Adult and Dislocated Worker programs), which may include a review comparing the services provided to male and female youth (41).
The balance of funding that goes to states is allocated to local workforce development areas on the same basis that Youth Activities funds are allocated to states, to take into account the relative numbers of unemployed individuals and low-income youth in the area compared to other local areas of the state. In addition, the law includes provisions for minimum (90% of the average allocation for the preceding two years) and maximum (130% of the average allocation for the preceding two years) funding that goes to local areas (42). Local areas may reserve no more than 10% of funds allotted under the program for administrative costs.
Elements of Local Programs
Job training and employment programs that are funded under WIOA and carried out by local WDBs are responsible for providing direct services to youth participants. The programs must be designed to include an objective assessment of the youth’s skills, and they must develop service strategies for these youth that are linked to employment goals (43). These service strategies must be directly linked to one or more of the indicators of performance for the program and they must identify career pathways that include both education and employment goals. Each local program for youth must also provide specific services, or elements. Table 3 shows the 14 elements required under WIOA. WIOA amended some of these elements and added some new ones. The table is organized based on whether the elements are targeted for educational achievement, employment services, linkages between educational achievement and employment services, leadership development activities, additional support for youth services, and other activities.

Table 3: Elements of Youth Programs as Specified Under WIOA
Local boards must provide each youth with information on the full array of applicable or appropriate services available through the local board, other eligible providers, or one-stop partners, and they must also refer youth to appropriate training and educational programs, among other activities (44). In addition, at least 20% of the funds allocated to the local area must be used to provide youth (whether they are in school or not) with paid and unpaid work experiences that have academic and occupational education as a component (45).
Although local boards have to make all program elements available to youth, each individual youth does not need to participate in all elements. Further, a local program that receives Youth Activities funding is not required to provide all program elements with WIOA funds; however, these other activities would have to be provided by a partner organization, and the other activities must be closely coordinated with the local programs. The program must have an agreement in place if it partners with another organization to ensure that a program element will be offered by that organization. In practice, this means that youth program case managers must contact and monitor the other provider to ensure the activity is of high quality and beneficial to the youth participant (46).
Participants
As shown in Table 4, WIOA specifies that youth are eligible for the Activities program if they are ages 14 through 24. In addition, local workforce development areas (and states) must use no less than 75% of funds for serving out-of-school youth. Up to 5% of the in-school youth in a local area may be eligible because they require additional assistance to complete an educational program or to secure or hold employment. A local workforce development area (or state) may adjust the share of out-of-school youth to 50% if the state determines it will be unable to use a certain share of funding to serve these youth (47). WIOA requires in-school youth generally and two groups of out-of-school youth to be low-income, and enables up to 5% of these youth to not meet the income criteria (48). Youth ages 18 through 21 may enroll in the Youth Activities program or Adult Activities program, or may co-enroll in both programs (49).
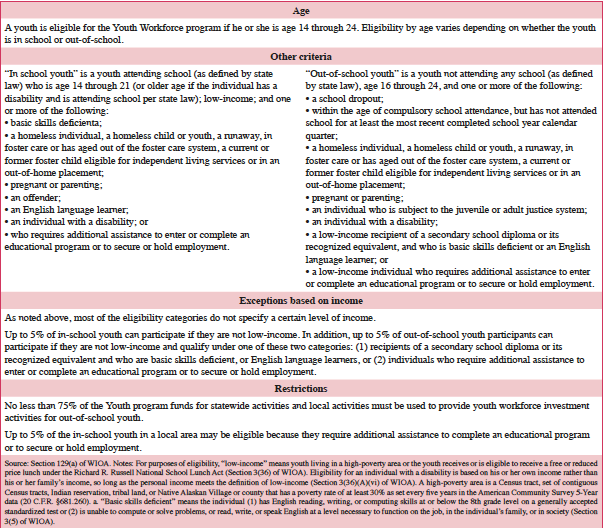
Table 4: Youth Program Eligibility Under WIOA
Performance
WIOA established six primary indicators of performance for the Youth program that superseded the WIA performance measures. These six primary indicators apply to all youth, regardless of age, and went into effect at the beginning of PY2016 (50):
• percentage of program participants who are in education or training activities, or in unsubsidized employment, during the second quarter after exit from the program;
• percentage of program participants who are in education or training activities, or in unsubsidized employment, during the fourth quarter after exit from the program;
• median earnings of program participants who are in unsubsidized employment during the second quarter after exit from the program;
• percentage of program participants who obtain a recognized postsecondary credential, or a secondary school diploma or its recognized equivalent (51), during participation in or within one year after exit from the program;
• percentage of program participants who, during a program year, are in an education or training program that leads to a recognized postsecondary credential or employment and who are achieving measurable skill gains toward such a credential or employment; and indicators of effectiveness in serving employers (52). States are required to reach an agreement with DOL, in conjunction with the Department of Education (ED), about the levels of performance for each state. These levels of performance are to be based on specified factors, including how the levels compare with other states’ adjusted levels of performance. Further, states are to ensure the levels are adjusted using an objective statistical model established by DOL (53).
The following sections of the report discuss, in less detail, additional programs for youth that are authorized under WIOA.
Job Corps (54)
Overview and Purpose
The Job Corps program is carried out by the Office of Job Corps within DOL’s Employment and Training Administration, and consists of residential centers throughout the country. The purpose of the program is to provide disadvantaged youth with the skills needed to—obtain secondary school diplomas or recognized postsecondary credentials leading to successful careers in in- demand industry sectors or occupations or the Armed Forces; or enroll in postsecondary education, including apprenticeship programs (55).
Program Structure
Currently, 125 Job Corps centers are in operation, and there is at least one center in every state and Puerto Rico (56). Of these 125 centers, 26 are known as Civilian Conservation Corps Centers, which are operated by the U.S. Department of Agriculture’s Forest Service, through an interagency agreement with DOL. Programs at these sites focus on conserving, developing, or managing public natural resources or public recreational areas. Most Job Corps centers are located on property that is owned or leased long-term by the federal government. Job Corps campuses include dormitories, classrooms, workshops for various trades, health centers, a cafeteria, a career services building, and administrative buildings. In addition to WIOA and its regulation, centers are to follow detailed guidelines about all aspects of the program as they are outlined in the program’s extensive policy guidance, known as the Policy and Requirements Handbook (57).
As specified under WIOA, Job Corps centers may be operated by a federal, state, or local agency; an area career and technical education school, or residential vocational school; or a private organization. Authorization for new Job Corps centers is contained in appropriations law. DOL initiates a competitive process seeking applicants that are selected based on their ability to coordinate activities in the workforce system for youth; ability to offer career and technical training opportunities that reflect local employment opportunities; relationships with the surrounding communities, employers, and other stakeholders; and (where applicable) past performance. Additionally, under WIOA, an entity applying to operate a center must submit to DOL certain information, such as how employment, education, and other opportunities offered at the center will reflect state and local employment opportunities and a description of the entity’s strong fiscal controls in place, among other information. WIOA specifies the contract may be for up to a two-year period with up to three one-year renewal periods (58).
Services for Students
While at a Job Corps center, students receive the following services:
• education programs, including English language acquisition programs;
• career and technical education, work experience, and work-based learning; and
• recreational activities, physical rehabilitation and development, driver’s education, and counseling, which may include information about financial literacy.
Youth also receive personal allowances while in the program and transition allowances as they are leaving the program. WIOA specifies that these transition allowances are to be incentive- based to reflect the graduate’s completion of academic, career and technical education or training, and attainment of recognized postsecondary credentials (59).
Students tend to experience the program in four stages (60). First, students learn about the program and center through orientation sessions and other outreach efforts conducted by the center and its contractor for outreach and admissions. Second, students who decide they want to pursue the program and are selected to continue on in with career preparation activities in the first few weeks of enrolling in the program. Third, students who continue on focus on career development activities. During this period, students learn and demonstrate career technical, academic, and employability skills. Training focuses on academic subject matters and how they are applied to specific trades or occupations. Students who did not graduate from high school can pursue a high school diploma or GED. Finally, students participate in a period of career transition, in which they receive placement services that focus on transitioning them in full-time jobs that are related to their career and technical training and pay wages that allow them to be self-sufficient, or placing them in higher education or advanced training programs, including apprenticeship programs.
For one year after exiting the program, Job Corps must provide graduates with services that include transition support and workplace counseling. Some graduates may go on to participate in an advanced career training program. These students continue to remain in the program for another year while obtaining additional training and education, such as an Associate’s Degree (61).
DOL contracts with entities—known as outreach and admissions (OA) contractors (though not referred to in the law as such)—to recruit students to the program. OA contractors seek out potential applicants, conduct interviews with applicants to identify their needs and eligibility status, and identify youth who are interested and likely Job Corps participants. Similarly, DOL contracts career transition services (CTS) providers—organizations that enter into a contract or other agreement with Job Corps—to provide placement services for graduates and, to the extent possible, former students. OA and CTS providers work closely with Job Corps centers, and in some cases are operated by the same organizations.
Community Engagement
Each Job Corps center director must establish relationships with employers, applicable one-stop centers and local boards, entities carrying out relevant apprenticeship programs and youth programs, and other stakeholders (62). Each center must establish a workforce council, made up of private sector employers who must have substantial management and other responsibilities and represent businesses with employment opportunities for youth in the program; representatives of labor organizations (where present) and representatives of employees; and Job Corps students and graduates (63). A majority of the members must be employers. The council must work with local workforce development boards and review local market information to provide recommendations about the center’s education and training offerings.
Allocations
DOL enters into contracts with nonprofit and for-profit organizations to operate the centers. Contracts are competitively awarded to organizations based on ranked scores, in conjunction with other factors. The contract period is two years, with three one-year-option renewals. DOL transfers funding for Civilian Conservation Centers to the U.S. Department of Agriculture (USDA) under an interagency agreement.
Participants
Job Corps participants must be ages 16 through 24 (64), low-income, and be one or more of the following: (1) basic skills deficient; (2) a school dropout; (3) homeless, a runaway, or a foster child (including an individual who was in foster care and has aged out of foster care); (4) a parent; (5) victims of a severe form of trafficking, as defined by the Trafficking Victims Protection Act; or (6) an individual in need of additional education, vocational training, or intensive counseling and related assistance in order to participate in regular schoolwork or to secure and maintain employment. A veteran is eligible if he or she meets the eligibility criteria; however, the income requirement does not apply if the veteran’s income earned in the military (within the six-month period prior to applying for the program) exceeds the income limit (65).
Job Corps centers take additional factors into consideration when selecting participants, such as whether the program can best meet their educational and vocational needs and whether the youth can engage successfully in group situations and settings. The applicant must also pass a background check that is conducted in accordance with applicable state and local laws (66). WIOA prohibits an individual from being denied a position in the Job Corps program solely on the basis of his or her contact with the criminal justice system, except that an individual can be denied a position if he or she has been convicted of a felony consisting of murder (as described in Title 18 of the U.S. Code), child abuse, or a crime involving rape or sexual assault (67). Each Job Corps center must develop standards for student conduct and implement what is known as a zero tolerance policy for offenses related to violence and drug and alcohol use, and selected other behaviors. Students are dismissed from the program if they violate this policy.
Students are to be assigned to the center that offers the type of career and technical education and training that he or she selects (unless the parent or the guardian of an enrollee under 18 objects). Among the centers that offer such education and training, the enrollee is to be assigned to the one closest to his or her home (68). No more than 20% of participants may live off the grounds of the Job Corps center (69).
WIOA specified that no individual may be enrolled in Job Corps for more than two years, except when completing an advance training program that would require the individual to participate for more than an additional year (as permitted for such a program); (2) an individual with a disability who would reasonably be expected to graduate, if allowed to participate for up to an additional year; and (3) in the case of an individual who participates in national service (as authorized by the Civilian Conservation Corps program) who may extend enrollment to equal the period of such national service (70).
Performance
WIOA directs DOL to establish expected levels of performance for the program and individual centers that relate to each of the six primary indicators of performance for the Youth Workforce Activities program. These indicators include (1) entry into education, training, or unsubsidized employment (during both the (a) second quarter and (b) fourth quarter after exiting the program); (2) median earnings; (3) obtaining a recognized postsecondary credential or secondary school diploma or its equivalent; (4) participation in an education or training program that leads to a credential or employment; and (5) program effectiveness in serving employers (71).
WIOA further specifies performance measures for recruiters (outreach and admissions) and CTS contractors. The OA performance measures pertain to recruitment and performance of students, as well as some of the same information that is to be included in a DOL report to Congress (72). This report must include information on the performance of each center, the program overall, and the OA and CTS contractors; demographic information on enrollees; the number of graduates who entered the Armed Forces, apprenticeships, unsubsidized employment, and postsecondary education; average wage of graduates; total cost per enrollee and graduate; information regarding the state of Job Corps facilities and buildings; and information regarding the national and community service activities of students, particularly those enrolled at Civilian Conservation Centers, among other information (73).
Performance Oversight
WIOA designates centers as high-performing based on their ranking and performance under the primary indicators of performance for youth. It also enables the operator of a high-performing center to compete in any competitive selection process carried out for an award to operate such center (74).
WIOA specifies that a Job Corps center operator failing to meet expected performance levels can be placed under a performance improvement plan (PIP). PIPs are documented plans that outline deficiencies in program performance, corrective actions, and targets for improvement. The plan is to encompass certain actions taken by DOL during a one-year period, including providing technical assistance to the centers; changing the career and technical training offered at the center; changing the management staff of the center; replacing the operator of the center; reducing the capacity of the center; relocating the center; or closing the center. WIOA also enables DOL to establish additional PIPs when a Job Corps center fails to meet performance requirements. These discretionary PIPs have to include the actions described above (75).
WIOA provides that DOL may not renew the agreement with a center operator if in the most recent two preceding years for which data are available the center is ranked in the lowest 10% of centers and fails to achieve an average of 50% or higher in the expected levels of performance under each of the primary indicators of performance for eligible youth in the program (76). The law allows DOL to renew an agreement with these centers (for up to two years and if in the best interest of the program) under certain circumstances (e.g., performance is due to circumstances beyond the operator’s control, etc.), and specifies standards that all centers must meet for agreements to be renewed (e.g., satisfactory record of integrity and business ethics, etc.). DOL must inform Congress of such renewals. WIOA specifies that DOL must select another entity to operate a Civilian Conservation Center if it fails to meet the expected levels of performance relating to the primary indicators of performance or fails to improve performance after three program years (77).
Prior to the closure of any Job Corps center, DOL must ensure (1) that the proposed decision to close the center is announced in advance to the general public through publication in the Federal Register or other appropriate means; (2) that a reasonable comment period, not to exceed 30 days, is established for interested individuals to submit written comments to the Secretary; and (3) that the Member of Congress who represents the district in which a center is located is notified within a reasonable period of time in advance of any final decision to close the center.
Finally, WIOA directs DOL to provide for a third-party evaluation of the program every five years, and to submit the results to Congress. The evaluation must address the general effectiveness of the program in relation to its costs; the effectiveness of the performance measures for the program; the effectiveness of the structure and mechanisms for delivering services; the impact of the program on the community, businesses, and participants involved; the extent to which the program and activities meet the needs of various demographic groups, and other such factors that may be appropriate (78).
Financial Oversight
WIOA requires DOL to prepare and submit reports to Congress that include information about implementing financial oversight measures suggested in a 2013 DOL IG report about oversight of Job Corps funding (79), a description of any budgetary shortfalls in the period covered by the report, and an explanation for approving contract expenditures that are in excess of the amount specified under a contract. The reports are to be provided every six months for an initial three-year period, then annually for another two years. WIOA further requires DOL to submit an additional report to Congress if the program has a budget shortfall, including an explanation of how the shortfall will be addressed. The report must be submitted within 90 days after the shortfall is identified (80).
YouthBuild (81)
Overview and Purpose
In 2007, YouthBuild was transferred from the Department of Housing and Urban Development to DOL under the YouthBuild Transfer Act (P.L. 109-281). As stated in WIOA, the purpose of YouthBuild is to (1) enable disadvantaged youth to obtain the education and employment skills necessary to achieve economic self-sufficiency in occupations in demand and post-secondary education and training opportunities; (2) provide disadvantaged youth with opportunities for meaningful work and service to communities; (3) foster the development of employment and leadership skills and commitment to community development among youth in low-income communities; (4) expand the supply of permanent affordable housing for homeless individuals and low-income families by utilizing the energy of disadvantaged youth; and (5) improve the quality and energy efficiency of community and other nonprofit and public facilities, including those facilities that are used to serve homeless and low-income families (82).
Program Structure
DOL competitively awards YouthBuild funds to organizations that carry out the program in cooperation with subgrantees or contractors or through arrangements made with local education agencies and certain other entities. Entities that are eligible to apply for funding include a public or private nonprofit agency or organization, including a consortium of such agencies or organizations. Specifically, such entities may include community-based or faith-based organizations; entities that carry out activities authorized under certain other parts of WIOA, such as a local workforce development board; community action agencies; state or local housing development agencies; an Indian tribe or agencies primarily serving Indians; state or local youth service or conservation corps; or any other entity eligible to provide education or employment training under a federal program (83).
While in the program, youth participate in a range of education and workforce investment activities, as listed in Table 5. These activities include instruction, skill building, alternative education, mentoring, and training in rehabilitation or construction of housing. Notably, any housing unit that is rehabilitated or reconstructed may be available only for rental by, or sale to, homeless individuals or low-income families; or for use as transitional or permanent housing to assist homeless individuals achieve independent living. All educational programs, including programs that award academic credit, and activities supported with YouthBuild funds must be consistent with applicable state and local educational standards.
At least 40% of the time, youth must participate in certain work and skill development activities (these activities are denoted by footnote “a” in Table 5). At least an additional 50% of the time, participants must be engaged in education and related services and activities designed to meet their educational needs (these activities are denoted by footnote “b” in Table 5). If approved by the DOL Secretary, training and supports may be provided in additional in-demand industry sectors or occupations. This is consistent with a 2012 regulation for the program that enables grantees to expand their occupational skills training beyond construction skills training; however, all programs must still provide training in the construction trades (84).
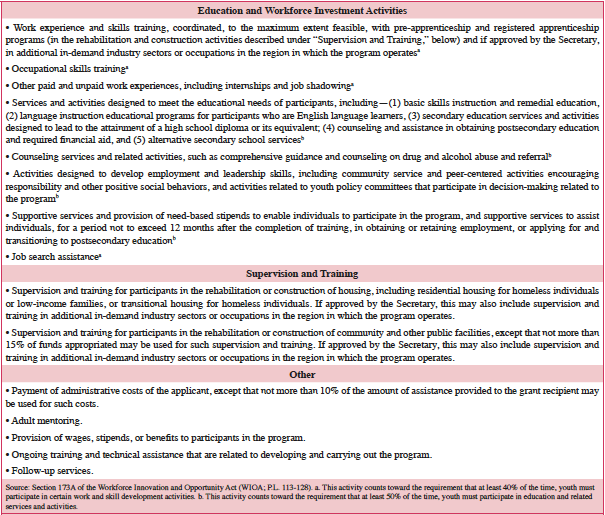
Table 5: Eligible Activities Funded by YouthBuild as Specified Under WIOA
Participants
Youth are eligible for the program if they are (1) ages 16 through 24; (2) a member of a low- income family, a youth in foster care, a youth offender, an individual with a disability, a child of incarcerated parents, or a migrant youth; and (3) a school dropout. However, up to 25% of youth in the program are not required to meet the income or dropout criteria, so long as they are basic skills deficient despite having earned a high school diploma, GED, or the equivalent; or have been referred by a high school for the purpose of obtaining a high school diploma.
Allocations
Grants are competitively awarded to organizations based on ranked scores, in conjunction with other factors, such as the applicant’s potential for developing a successful YouthBuild program; the need for the program in the community; the applicant’s commitment to providing skills training, leadership development, and education to participants; regional distribution of grantees; and the applicant’s coordination of activities to be carried out with certain other stakeholders, including employers, one-stop partners, and national service and other systems; among other criteria.
DOL makes awards for three years (two years of program operations with a one-year period of follow-up). Applicants must provide cash or in-kind resources equivalent to at least 25% of the grant award amount as matching funds. Prior investments and federal resources do not count toward the match.
Performance
WIOA requires YouthBuild grantees to meet the primary indicators of performance for eligible youth described in the Youth Activities program. Specifically, these indicators pertain to entry into education, training, or unsubsidized employment (both two and four quarters after exiting the program); median earnings; obtaining a recognized postsecondary credential or secondary school diploma or its equivalent; participation in an education or training program that leads to a credential or employment; and program effectiveness in serving employers (85).
Reentry Employment Opportunities Program (86)
Overview and Purpose
Grants to provide education and employment activities for youth offenders have been funded by DOL since FY1999 (87). Under WIA, these grants were made part of the Reintegration of Ex- Offenders program. Funding for the program was authorized under both WIA and Section 112 (Responsible Reintegration of Offenders) of the Second Chance Act (P.L. 110-199), enacted on April 9, 2008. The Second Chance Act authorizes DOL to make grants to nonprofit organizations for the purpose of providing mentoring, job training and job placement services, and other comprehensive transitional services to assist eligible offenders ages 18 and older in obtaining and retaining employment. Following the enactment of WIOA, Congress has appropriated funding for the program, now known as the Reentry Employment Opportunities program, under the authority of Section 169 of WIOA and the Second Chance Act. Section 169 authorizes evaluations and research.
The youth component of the REO program (and its predecessor programs) has been comprised of related initiatives that seek to assist youth offenders and youth at risk of dropping out (or who have dropped out) with pre-release, mentoring, housing, case management, and employment services; to reduce violence within persistently dangerous schools through a combination of mentoring, educational, employment, case management, and violence prevention strategies; and to provide alternative education and related services for youth at risk of involvement with the justice system (88). Currently , the program supports education and reentry initiatives.
Program Structure
The earliest DOL initiatives for youth offenders, from FY1999 through FY2004, operated under what is known as the Youth Offender Demonstration Project (YODP) (89). The pilot funded 52 grantees to assist youth at risk of court or gang involvement, youth offenders, and gang members ages 14 to 24 in finding long-term employment.
The more contemporary grant programs for youth offenders have funded multiple projects in recent years that have a focus similar to the earlier projects under YODP. These projects have included (1) education-related grants; (2) apprenticeship and related grants under grants collectively called Categorical Grants (Youth Offender Registered Apprenticeship, Alternative Education, and Project Expansion Grants); (3) grants that focus on reentry, including Beneficiary- Choice Demonstration, High Growth Youth Offender Initiative, Planning, State/Local Implementation, and Replication Grants; and (4) grants that focus on community service, including Civic Justice Grants and Serving Young Adult Ex-Offenders through Training and Service Learning. Grantees have included local and state governments, nonprofit organizations, including faith-based organizations; school districts; and community colleges (90).
FY2016 appropriations support an education-related grant, the Pathways to Justice Careers program. Funds have been provided to five nonprofit organizations and two local governments to support youth 16 to 21 who are at risk of dropping out of high school, becoming involved in the juvenile justice system, or already have had involvement in the juvenile justice system. The program focuses on providing mentoring—by individuals in justice-related positions (e.g., police officers, fire fighters, lawyers, etc.)—and career training that uses a career pathways model for youth who are in school. A career pathway model includes a sequence of rigorous academic and career and technical education courses that result in educational and skills credentials. The program also aims to ensure that youth graduate from high school and/or pursue further training or post-secondary education.
A reentry program, Reentry Demonstration Projects for Young Adults, is also funded with FY2016 appropriations. These projects are designed to assist youth ages 18 to 24 who are reentering, with a focus on interventions such as mentoring, registered apprenticeships, family unification efforts, and other promising practices that focus on providing occupational training and credentials. DOL intends to conduct a rigorous evaluation of the seven grantees.
Participants
Each of the initiatives funded under the Reentry Employment Opportunities program (and its predecessor programs) have generally served select groups of at-risk youth. However, the projects generally serve youth ages 14 and older (or 18 or older) who have been involved with or have a high risk of involvement in gangs or the juvenile justice system or criminal justice system.
Allocations
As noted, grants have been competitively awarded to entities such as community-based organizations and state and local juvenile justice agencies, based on ranked scores and other factors, depending on the project. Only schools that meet the criteria of “persistently dangerous,” as specified by the states and as permitted under the Elementary and Secondary Education Act were eligible to apply for funds under the Persistently Dangerous Schools Initiative (91). Allocations have varied for each of the projects, but, generally, grantees have received grants of $1 million to $5 million for one or more years.
Performance
DOL has three performance measures for each REO initiative: (1) attainment of a degree or industry-recognized certificate for individuals age 18 or older; (2) literacy and numeracy attainment; and (3) out-of-school participants age 18 or older who are placed in unsubsidized jobs, post-secondary education, or occupational training (92).
References
1. CRS Report R42519, Youth and the Labor Force: Background and Trends, by Adrienne L. Fernandes-Alcantara
2. Two rules accompany the WIOA law. U.S. Department of Labor (DOL), Employment and Training Administration (ETA), “Workforce Innovation and Opportunity Act; Final Rule,” 81 Federal Register 56072, August 19, 2016; and U.S. Department of Labor, Employment and Training Administration and Department of Education, “Workforce Innovation and Opportunity Act; Joint Rule for Unified and Combined State Plans, Performance Accountability, and the One-Stop System Joint Provisions; Final Rule,” 81 Federal Register 56072, August 19, 2016. See other DOL, ETA resources: “Workforce Innovation and Opportunity Act,” http://www.doleta.gov/wioa/; Training and Employment Guidance Letter (TEGL) No. 19-14, “Vision for the Workforce System and Initial Implementation of the Workforce Innovation and Opportunity Act of 2014,” February 19, 2015; and TEGL No. 23-14, “Workforce Innovation and Opportunity Act (WIOA) Youth Program Transition.” See also, CRS Report R44252, The Workforce Innovation and Opportunity Act and the One-Stop Delivery System, by David H. Bradley.
3. Most workforce programs operate on a program year basis, which extends from July 1 of one year through June 30 of the following year.
4. For further information about youth prospects in the labor market, see CRS Report R42519, Youth and the Labor Force: Background and Trends, by Adrienne L. Fernandes-Alcantara; and CRS Report R40535, Disconnected Youth: A Look at 16 to 24 Year Olds Who Are Not Working or In School, by Adrienne L. Fernandes-Alcantara.
5. DOL, BLS, “Employment projections: Earnings and Unemployment Rates by Educational Attainment,” March 15, 2015, http://www.bls.gov/emp/ep_chart_001.htm.
6. Emily Richards and David Terkanian, “Occupational Employment Projections to 2024,” Monthly Labor Review, December 2015, pp. 9-10, http://www.bls.gov/opub/mlr/2013/article/pdf/occupational-employment-projections-to- 2022.pdf. (Hereinafter Emily Richards and David Terkanian, “Occupational Employment Projections to 2024.”) See also, Anthony P. Carnevale, Nicole Smith, and Jeff Strohl, Help Wanted: Projections of Jobs and Education Requirements through 2018, Georgetown University, Center on Education and the Workforce, June 2010, http://cew.georgetown.edu/JOBS2018/.
7. Northeastern University, Center for Labor Market Studies, The Consequences of Dropping Out of High School: Joblessness and Jailing of High School Dropouts and the High Cost for Taxpayers, October 1, 2009, http://iris.lib.neu.edu/cgi/viewcontent.cgi?article=1022&context=clms_pub; Paul E. Barton, One Third of a Nation: Rising Dropout Rates and Declining Opportunities, Educational Testing Services, February 2009, http://www.ets.org/ Media/Education_Topics/pdf/onethird.pdf; and Clive R. Belfield, Henry M. Levin, and Rachel Rosen, The Economic Value of Opportunity Youth, prepared for the Corporation for National and Community Service and the White House Council for Economic Solutions, January 2012, http://files.eric.ed.gov/fulltext/ED528650.pdf.
8. Unless otherwise noted, this section draws heavily on an archived report by the Congressional Research Service, Youth Employment: A Summary History of Major Federal Programs, 1933-1976. Available upon request.
9. John H. Bremner, Tamara K. Hareven, and Robert M. Mennel, eds., Children & Youth in America, Vol. II: 1866- 1932, Parts 1-6 (Cambridge, MA: Harvard University Press, 1971), pp. 687-749.
10. Much of this section on YEDPA was drawn from Charles L. Betsey, Robinson G. Hollister, and Mary R. Papageorgiou, eds., Youth Employment and Training Programs: The YEDPA Years, National Research Council, Washington, DC, 1985, http://www.eric.ed.gov/ERICWebPortal/custom/portlets/recordDetails/detailmini.jsp?_nfpb= true&_&ERICExtSearch_SearchValue_0=ED265245&ERICExtSearch_SearchType_0=no&accno=ED265245. (Hereinafter, Betsey, Hollister, and Papageorgiou, Youth Employment and Training Programs.)
11. A fourth program, the Young Adult Conservation Corps (YACC), was operated by the Department of Agriculture and Department of the Interior, in cooperation with DOL, and targeted unemployed youth ages 16 to 23 who were not necessarily disadvantaged. This program operated year-round and was separate from a similarly named program, the Youth Conservation Corps (YCC). YCC was permanently authorized by the Youth Conservation Corps Act of 1970 (P.L. 91-378) and continues to operate.
12. Other parts of YEDPA required close coordination with the school system. According to an assessment of the act’s implementation, the schools maintained their focus on in-school youth and provided essentially the same set of educational services as usual. The lack of influence of YEDPA on schools may be largely attributed to the schools’ resistance to allocating services according to income and the schools’ perception that their mission was exclusively to educate students. Betsey, Hollister, and Papageorgiou, Youth Employment and Training Programs, pp. 84-87.
13 . Unless otherwise noted, this section was drawn heavily from an archived report by the Congressional Research Service, The Job Training Partnership Act: A Compendium of Programs. Available upon request.
14. Archived report by the Congressional Research Service, Job Training Partnership Act: Legislation and Budget Issues. Available upon request.
15. Archived report by the Congressional Research Service, The School-to-Work Opportunities Act. Available upon request.
16. For further information about the Adult and Dislocated Worker programs, see CRS Report RL33687, The Workforce Investment Act (WIA): Program-by-Program Overview and Funding of Title I Training Programs, by David H. Bradley.
17. Section 101(b)(1)(II) of WIOA.
18. Section 102(b)(1)(D) of WIOA.
19. Section 108(b)(9) of WIOA.
20. Section 107(b)(4)(ii) of WIOA.
21. Section 121(b)(1)(B) of WIOA. For further information, see CRS Report R44252, The Workforce Innovation and Opportunity Act and the One-Stop Delivery System, by David H. Bradley.
22. Section 189(g)(1)(A) of WIOA. Section 173(h)(2), which pertains to authorization for YouthBuild, states that notwithstanding Section 189(g), appropriations for any fiscal year for programs and activities carried out under this section are to be available for obligation only on the basis of a fiscal year.
23. Section 189(g)(2) of WIOA.
24. Title I, Chapter 2 of WIOA and 20 C.F.R. 20 C.F.R. §681.
25. Section 102 and Section 103 of WIOA.
26. Section 121 of WIOA.
27. Section 107(b) of WIOA.
28. Section 107(2)(iv) of WIOA.
29. Section 123(b) of WIOA. The regulations (20 C.F.R. §681.400) specify that local workforce development boards may choose to directly provide some or all youth workforce activities.
30. Section 107(d)(10(B) and Section 123 of WIOA.
31. 20 C.F.R. §681.700.
32. Section 129(c)(3)(C) of WIOA.
33. Section 107(b)(4)(A)(ii) of WIOA.
34 . 20 C.F.R. §681.100.
35. The outlying areas comprise the U. S. Virgin Islands, Guam, American Samoa, the Commonwealth of the Northern Mariana Islands, the Republic of the Marshall Islands, the Federated States of Micronesia, and the Republic of Palau. WIOA specifies that the Republic of Palau may not apply for funding during any period during which DOL and the Department of Education (ED) determine that a Compact of Free Association (COFA) is in effect and contains provisions for training and education assistance that prohibit the assistance provided under WIOA. COFA defines the relationship that Palau has entered into as an associated state agreement with the United States. No such determination prohibiting assistance to Palau has been made.
36 Section 127(1) of WIOA.
37. Section 127(b)(1)(B)(ii) of WIOA. In practice, funds for outlying are based on a formula determined by the Secretary that was used under WIOA. See, for example, DOL, ETA, Training and Employment Guidance Letter (TEGL) No. 17- 5, “Workforce Innovation and Opportunity Act (WIOA) Adult, Dislocated Worker and Youth Activities Program Allotments…. ” April 5, 2016.
38. The word “relative” means the number of individuals in a state compared to the total number in all states.
39. WIOA provides small state minimums such that no state receives less than the total of three-tenths of 1% of $1 billion that is allocated to states, or two-thirds of 1% of the excess if the allocation exceeds $1 billion.
40. Section 128(a) of WIOA.
41. Section129(b) of WIOA.
42. Section 128(b) of WIOA.
43. Section 129(c) of WIOA.
44. Section 129(c)(3) of WIOA.
45. Section 129(c)(3)(4) of WIOA
46. 20 C.F.R. §681.470. 47 A local area is exempt if it is in a state that (1) receives 90% of the allotment percentage for the preceding fiscal year for the Youth Activities program (per Section 127(b)(1)(C)(iv)(I)) or Adult program (Section 132(b)(1)(B)(iv)(I)); or (2) receives the small state minimum allotment under the Youth Activities program (per Section 127(b)(1)(C)(iv)(II)) or Adult program (per Section 132(b)(1)(B)(iv)(II). A local area that meets one of these two criteria would be able to decrease the funds used for out-of-school youth to 50% of their allotment (that is used for non-administrative costs) only if the state (1) determines that the local area will be unable to use at least 75% of the funds available due to a low number of out-of-school youth; (2) submits to the Secretary of DOL, on behalf of the local area, a request including a proposed percentage decrease (not less than 50%), and (3) a summary of the analysis about the determination. This request has to be approved by the Secretary.
48. Section 129(a)(4) of WIOA
49. 20 C.F.R. §681.430.
50. Section 116(A) of WIOA.
51. Program participants who obtain a secondary school diploma or its recognized equivalent are to be included in the percentage counted if, in addition to obtaining such diploma or its recognized equivalent, they have obtained or retained employment or are in an education or training program leading to a recognized postsecondary credential within one year after exit from the program.
52. The law specifies that DOL and the Department of Education are to jointly develop and establish one or more indicators of performance that indicate the effectiveness of the Youth program (and Adult and Dislocated Worker programs) in serving employers.
53. Section 116(b(3)(v) of WIOA.
54. Title I, Chapter 4, Subtitle C of WIOA and 20 C.F.R. §670.
55. Section 141 of WIOA.
56. DOL closed the Treasure Lake Job Corps Center in Oklahoma in 2014 and the Ouachita Civilian Conservation Center in Arkansas in 2016 due primarily to low performance. See, DOL, ETA, “Final Notice of Job Corps Center for Closure,” 79 Federal Register 61099, October 9, 2014; and DOL, ETA, “Final Notice of Job Corps Center for Closure,” 81 Federal Register 43250, July 1, 2016.
57. DOL, ETA, Office of Job Corps, Policy and Requirements Handbook, http://www.jobcorps.gov/Libraries/pdf/ prh.sflb. (Hereinafter, DOL, ETA, Office of Job Corps, Policy and Requirements Handbook.)
58. Section 147(a) of WIOA.
59. Section 150 of WIOA.
60. DOL, ETA, Office of Job Corps, Policy and Requirements Handbook: and U.S. Government Accountability Office, Job Corps Better Targeted Career Training and Improved Preenrollment Information Could Enhance Female Residential Student Recruitment and Retention, GAO-09-470, June 2009.
61. Section 148(c) of WIOA.
62. Section 153 of WIOA.
63. Section 154(c) of WIOA.
64. No more than 20% of participants may be ages 22 through 24 on the date of enrollment. The age limit may be waived by DOL, in accordance with DOL regulations, for individuals with a disability.
65. Section 145 of WIOA.
66. Section 145(a) of WIOA.
67. Section 145(b) of WIOA. 68 Section 145(d) of WIOA. 69 Section 147(b) of WIOA. 70 Section 146 of WIOA.
71. Section 159(c)(1) of WIOA.
72 . Section 159(c)(2) and Section 159(c)(3) of WIOA.
73. Section 159(d) of WIOA.
74. Section 147(b) of WIOA.
75. Section 159(f) of WIA and WIOA.
76. Section 147(g) of WIOA.
77. Section 147(g)(4) of WIOA.
78. Section 161(b) of WIOA.
79. DOL, Office of Inspector General, The U.S. Department of Labor’s Employment and Training Administration Needs to Strengthen Controls Over Job Corps Funds.
80. Section 161(a) of WIOA.
81. Title I, Chapter 4, Subtitle D, Section 171 of WIOA. 82 Section 171(a) of WIOA (Section 173A(a) of WIA). 83 Section 173(b) of WIOA (Section 173A(b) of WIA).
84. U.S. Department of Labor, Employment and Training Administration, “YouthBuild Program Final Rule,” 77 Federal Register 9112, February 15, 2012.
85. Section 171(c) of WIOA (and Section 173(c) of WIA).
86. Title I, Chapter 4, Subtitle D, Section 169 of WIOA.
87. This program was known as the Youth Offender Pilot Program, and funded 14 communities that provided educational, employment, re-entry, and other services to youth.
88. This is based on a review of initiatives funded by the Reintegration of Ex-Offenders program. DOL, ETA, Youth Services Discretionary Grants, http://www.doleta.gov/Youth_services/Discretionary.cfm.
89. The earliest funding for the program was authorized under Title IV of the Job Training Partnership Act. See U.S. Department of Labor, Employment and Training Administration, Notice Inviting Proposals for Youth Offender Demonstration Projects, August 28, 1998, http://www.doleta.gov/grants/sga/01-101sga.cfm.
90. For a list of grantees and grant funding amounts, see DOL, ETA, Youth Services Discretionary Grants, http://www.doleta.gov/grants/.
91. ESEA requires each state receiving funds under the act to establish and implement a statewide policy requiring that a student attending a persistently dangerous school, as determined by the state in consultation with a representative sample of local education agencies (LEAs), or a student who becomes a victim of a violent criminal offense on school grounds be allowed to attend a safe school within the LEA.
92. CRS correspondences with DOL, ETA, December 2016.