Susanne Bügel1, Balz Frei2
1. Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark;
2. Linus Pauling Institute and Department of Biochemistry & Biophysics, Oregon State University, Corvallis, Oregon, USA.
Corresponding to: Susanne Bügel, MSc, PhD, Professor, Department of Nutrition, Exercise and Sports, University of Copenhagen, Rolighedsvej 26, 1958 Frederiksberg C, Denmark, Phone: +45 35 33 24 90, E-mail: shb@nexs.ku.dk
Care Weekly 2019;
Published online May 6, 2019, http://dx.doi.org/10.14283/cw.2019.4
Abstract
This narrative review describes the distinct nutritional needs of middle-aged and older adults in the European Union. Literature reviews were conducted to identify sources evaluating nutritional status and interventions relevant to these populations. Emphasis was placed on dietary guidelines, systematic reviews, and meta-analyses examining relevant macronutrients and micronutrients and important diseases or conditions related to aging (e.g. cardiovascular disease, infections, osteoporosis, cognition, immunity). Middle-aged and older adults in the European Union frequently do not obtain recommended amounts of key macronutrients and micronutrients necessary for maintaining health. In addition to the nutritional benefits of a healthful diet and contact with professionals to identify nutritional barriers, problem-solving techniques and micronutrient and macronutrient goals can improve the outcomes of dietary interventions in these individuals. Nutrition education programs, particularly those with specific recommendations, are effective for improving the nutritional status of these populations. For those who do not obtain adequate amounts of macronutrients and micronutrients from their diets, adhering to dietary guidelines and, when warranted, supplementation should be considered to improve nutritional status. The findings from randomized, controlled trials suggest that dietary interventions and supplementation can correct nutritional deficiencies and inadequacies that are important to the health of middle-aged and older adults. However, it is important to evaluate nutrient intake from the diet, supplementation, and fortified food to avoid exceeding tolerable upper intake levels of certain nutrients and limit potential adverse outcomes. Medical histories, medication use, dietary patterns, and other risk factors should be considered when recommending dietary improvements and supplements in these populations.
Key words: Aging, malnutrition, micronutrients, nutritional deficiencies.
Introduction
Life expectancies in European Union (EU) countries continue to rise, expanding the aging population. It has been estimated that by 2050 greater than 25% of the EU population will be ≥65 years of age, which has the potential to challenge the healthcare system (1). According to the European Health Report, important non-communicable diseases (NCDs) associated with aging include cardiovascular disease (CVD), certain cancers, and type 2 diabetes (1). Because these NCDs are the leading causes of early mortality in the EU, primary and secondary prevention efforts are needed to reduce NCD-related morbidity and mortality (1). To allow older individuals to live more independently and remain integrated in society, the World Health Organization’s agenda for preventing NCDs includes increasing physical activity, reducing tobacco and alcohol use, and reducing the risk of malnutrition, a condition in which many nutrient requirements are not met (2). The European Food Safety Authority (EFSA) reference intakes for selected micronutrients in adults >50 years of age are listed in Table 1 (3).
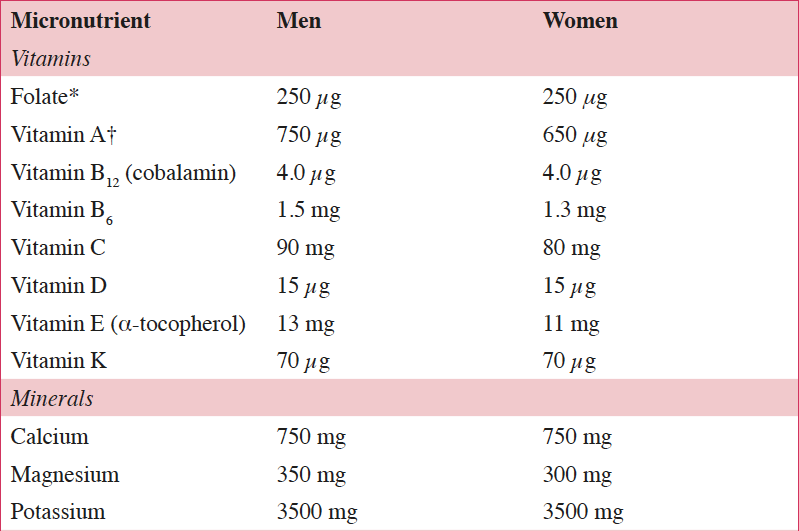
Table 1. European Food Safety Authority (EFSA) daily reference intakes for selected micronutrients in adults >50 years of age (3)
*1 μg of DFE equals 1 μg of food folate=0.6 μg of folic acid from fortified food=0.5 μg of a folic acid supplement; †1 μg RE equals 1 μg of retinol, 6 μg of β-carotene, and 12 μg of other provitamin A carotenoids; DFE, dietary folate equivalent; RE, retinol equivalent.
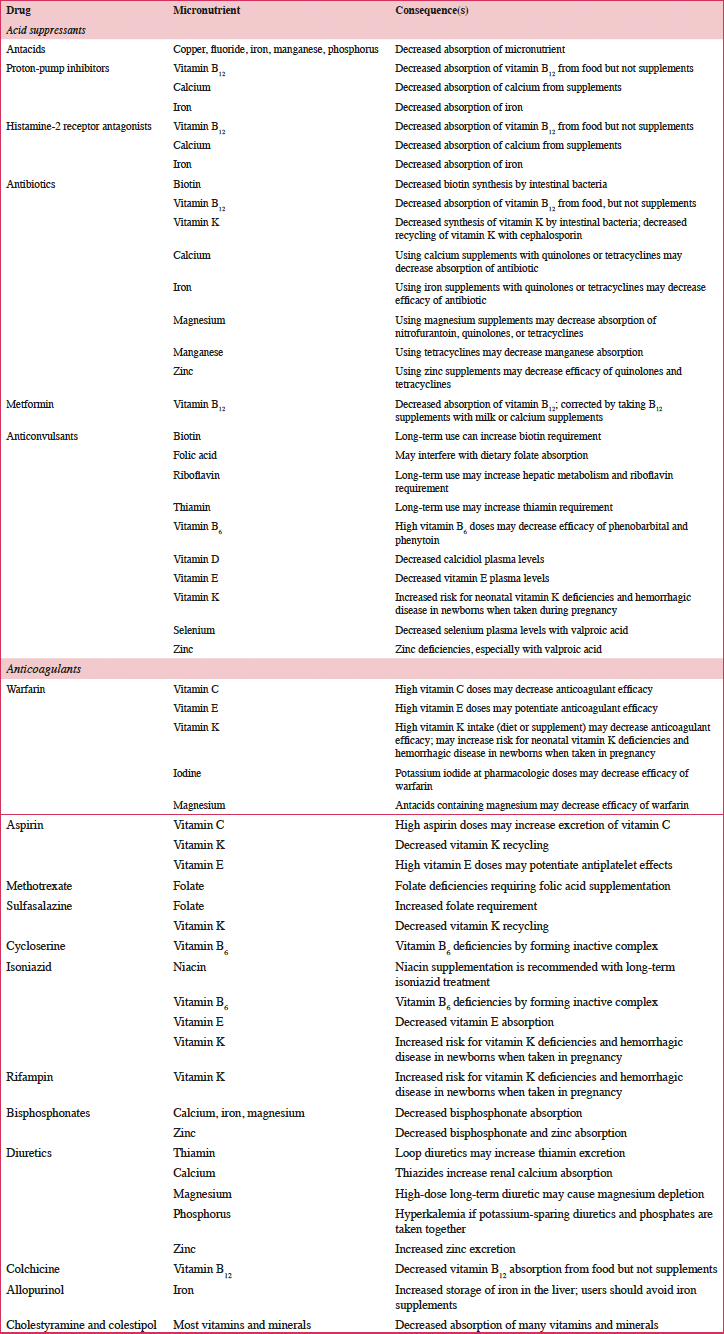
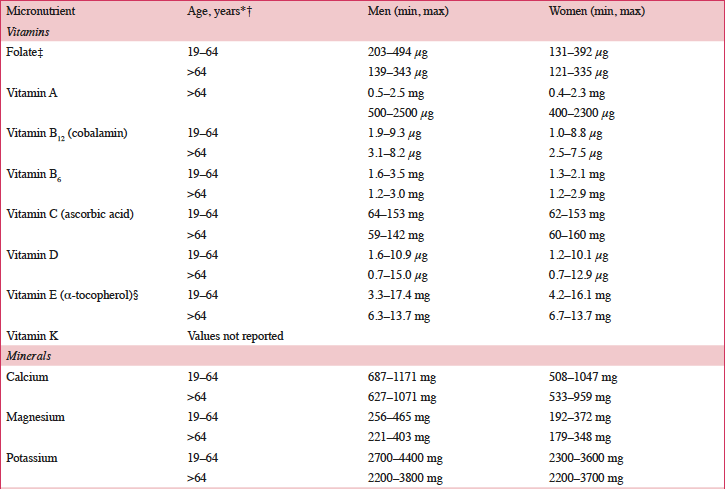
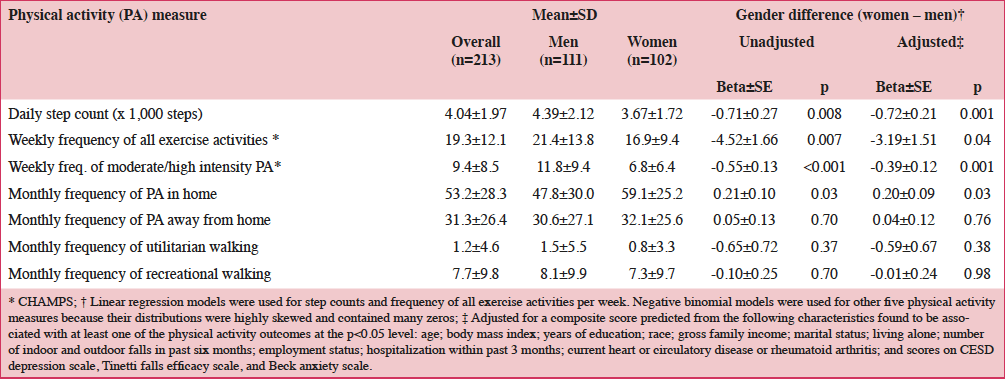
According to a systematic review of longitudinal data, risk factors for malnutrition include frailty, excessive polypharmacy, declining physical functioning and cognition, depression, dysphagia, and institutionalization (4). Multiple physical, socioeconomic, and cultural factors affect the nutritional status of older individuals, including changes in the ability to absorb nutrients, reduced appetite, and decreased ability to chew (4, 5). Furthermore, there are multiple drug-nutrient interactions that should be considered for this population (Table 2) (6). Insufficient energy intake associated with aging is complex and may involve chronic illnesses and reduced ability and desire to prepare and eat meals (7). Micronutrient intake below recommended amounts increases NCD risks (8). For example, micronutrient deficiencies can cause mitochondrial decay, a mechanism contributing to aging and development of diseases including cancer and neural decay (8). A relationship has been observed between the intake of certain micronutrients (i.e. vitamins D, B6, B12, and E and folate) and frailty in older adults (9). Values for ranges of nutrient intakes in the EU are described in Table 3 (10).
*Intakes reported for individuals 19–64 years of age for all countries except the following: Greece: 22±2 years of age; Hungary: ≥18 years of age; United Kingdom: 25–64 years of age; †Intakes reported for individuals >64 years of age for all countries except Hungary (>59 years of age); ‡Folate equivalent; 1 μg food folate=0.5 μg folic acid (PGA)=0.6 μg folic acid taken with meals; §RRR-α-tocopherol equivalent=mg α-tocopherol + mg β-tocopherol x 0.5 + mg y-tocopherol x 0.25 + mg α-tocotrienol x 0.33. DFE, dietary folate equivalent; PGA, pteroyl glutamic acid.
This narrative review describes the nutritional needs of middle-aged (50–64 years) and older (≥65 years) adults in the EU and interventions healthcare professionals should consider. Literature searches were conducted to identify sources that evaluated the nutritional status and interventions relevant to this population, with an emphasis placed on systematic reviews, meta-analyses, and dietary guidelines. Notably, some meta-analyses also included other ages (<50 years), but those that are included primarily assessed older individuals.
Protein
Aging leads to a loss of muscle mass and strength and poor physical performance (i.e. sarcopenia) that has been associated with macro- and micronutrient deficiencies, suggesting that a high-quality diet that includes optimal protein and nutrient intake combined with physical exercise can reduce this risk (11). Sarcopenia can increase the risk for falls, fractures, disability, loss of independence, and increased mortality (11). The prevalence of sarcopenia is approximately 1–29% in community-dwelling older adults and 10–33% in those living in long-term care and acute hospital settings (12).
Adequate protein intake is important to healthy aging, and higher intakes may be necessary to compensate for the difficulty of maintaining muscle mass; however, recommendations vary by country (13). The EFSA recommendation for protein intake for adults is 0.83 g/kg/d (3), yet, the PROT-AGE Study Group recommends protein intakes in older adults of 1.0–1.2 g/kg/d (13). The authors state that those with chronic diseases, severe illnesses, injury, or malnutrition may require higher intakes (i.e. 1.2–1.5 and 2.0 g/kg/d, respectively) (13). Higher protein intake can negatively impact kidney function in those with severe kidney disease not receiving dialysis; therefore, caution should be taken in this population (13). The Nordic Nutrition Recommendations set a tentative recommendation of 1.2–1.5 g/kg/d while stressing that adequate data do not exist to estimate an optimal protein intake (14).
Protein quality, timing of administration, whether to supplement with single amino acids, and the addition of physical exercise should be considered (13). Protein supplementation immediately following resistance training exercise is beneficial for muscle mass and strength (13). A recent meta-analysis of randomized, controlled trials (RCTs) conducted with whey-, leucine-, and casein-based protein supplements combined with resistance training reported that elderly individuals adhering to this regimen improved lean body and appendicular mass, body fat and mass, muscle strength, and mobility (15). However, the International Sarcopenia Initiative stated that protein supplements alone or combined with resistance training have shown inconsistent effects on muscle mass and function in individuals ≥50 years of age (12).
Dietary fiber
Dietary fiber is critical to maintaining proper laxation and a healthy microbiome and is involved in many physiological activities including protecting against CVD (5). EFSA recommends that older adults consume 25 g/d of total fiber (3), but few meet this goal (5). The European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) recommendations for managing dyslipidemia include consuming 45% to 55% of energy from carbohydrates, including fruit, vegetables, whole grains, legumes, and nuts, and 25-40 grams of total dietary fiber, such as β-glucan from oat and barley (16).
A meta-analysis of 67 RCTs reported that consuming high-fiber diets significantly reduced total and low-density lipoprotein (LDL) cholesterol, but not high-density lipoprotein cholesterol (17). Other meta-analyses of RCTs reported that using different fiber supplements significantly reduced diastolic blood pressure, an effect that was more pronounced in adults >40 years of age compared with younger adults (18), and significantly reduced glycated hemoglobin in middle-aged and older adults with type 2 diabetes (19).
Omega-3 fatty acids
Omega-3 fatty acids have anti-inflammatory properties, and higher intakes have been linked to reductions in CVD risk and cognitive impairment (5). Omega-3 fatty acids cannot be efficiently converted from dietary alpha-linolenic acid in the body; therefore, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) often do not reach adequate levels. Dietary sources of omega-3 fatty acids include oily fish, flaxseed, and walnuts (5). EFSA recommends that adults consume 0.5% of their energy in the form of alpha-linolenic acid and 250 mg/d EPA plus DHA (3). ESC and EAS recommendations include 2–3 g/d of long-chain omega-3 fatty acid supplements to reduce triglycerides and regular consumption of fish and nuts for preventing CVD (20).
Meta-analyses of RCTs conducted in adults with or without cardiovascular comorbidities (e.g. coronary heart disease, heart failure) receiving DHA and EPA through the diet or supplementation reported a greater risk reduction among individuals with elevated triglyceride and LDL cholesterol levels (21) and significantly improved brain natriuretic peptide and serum norepinephrine levels (22). The Multicenter Osteoarthritis Study, a prospective cohort study of individuals (mean age: 60 years) with, or at high risk for, knee osteoarthritis (OA) reported that individuals with high plasma omega-3 fatty acid levels, especially DHA, had less patellofemoral cartilage loss (23). Well-controlled RCTs are necessary to support using omega-3 fatty acid supplements for OA.
Due to the association between inadequacies in omega-3 fatty acid status and cognitive decline, a number of studies have been conducted to evaluate the effects of omega-3 fatty acids on cognitive functioning. Meta-analyses of observational studies and RCTs conducted in middle-aged and older adults reported significant improvements in cognitive functioning (i.e. attention, executive functioning, memory), but no improvements in other cognitive parameters were observed (24, 25). A more recent meta-analysis of omega-3 fatty acid trials in adults (>40 years of age) did not observe improvements in cognitive functioning (26).
Folate and vitamins B6 and B12
Folate is a B-vitamin involved in the metabolism of nucleic acid precursors, DNA methylation, homocysteine metabolism, and cognition (5). Vitamin B6 is essential for many enzymatic reactions involved in protein metabolism, while vitamin B12 is involved in folate metabolism and neurological functioning. Deficiencies in vitamin B12 can cause peripheral neuropathy and cognitive dysfunction (3, 5). EFSA recommends that adults consume 250 μg/d folate, 4.0 μg/d vitamin B12, and 1.5 mg/d and 1.3 mg/d vitamin B6, in men and women, respectively (3). Deficiencies and inadequacies in folate and vitamins B6 and B12 can increase homocysteine concentrations, weaken immunity, and increase risk of CVD, stroke, cognitive dysfunction, depression, osteoporosis, and fracture risk (5, 27). In the EU up to 20% of older adults (>64 years) do not obtain adequate amounts of vitamin B12, and a substantial proportion (17–46%) do not achieve adequate intake of folate (28). Older adults may have difficulty extracting vitamin B12 from natural food sources due to an age-related decline in gastric acid secretion (5), which reduces the ability of vitamin B12 to bind to intrinsic factor (29).
Meta-analyses of folic acid supplement RCTs in middle-aged and older adults reported significant risk reductions in stroke and CVD-related events (30) and significant reductions in plasma homocysteine concentration, with further reductions when vitamin B12 was co-administered (31).
Folic acid and vitamin B12 supplementation may also improve bone health, primarily due to the effects on homocysteine levels (32). However, an RCT conducted in older individuals (≥65 years) with elevated homocysteine levels supplemented with folic acid and vitamin B12 reported no reduction in fracture risk, aside from a sub-group of individuals >80 years of age (32).
A meta-analysis of case-control studies reported significant associations between higher folate intake and decreased risk of head and neck squamous cell carcinoma (33). Despite these potential benefits, excessive intake of synthetic folic acid can increase the risk of certain cancers (34). Therefore, it is important to determine an individual’s intake of dietary folate and folic acid from supplements and fortification to avoid reaching levels that can cause adverse health outcomes.
In meta-analyses of RCTs of folic acid in subjects ≥45 years of age without dementia (35) and vitamin B6 and B12 in subjects ≥40 years of age who were healthy or at risk for CVD (26), no improvements in cognitive functioning were found. Another trial that administered folic acid and vitamin B12 to older adults (60–74 years) reported significant improvements in overall cognitive functioning and immediate and delayed recall (36).
Vitamin A
Vitamin A is a fat-soluble vitamin found either as preformed vitamin A (retinol) in animal products such as liver and dairy, or as provitamin A carotenoids such as β-carotene in fruit and vegetables (3, 37). Vitamin A is involved in regulating cellular growth and differentiation and is required for healthy immune function and vision (3, 37). EFSA recommends that adult men and women consume 750 μg/d and 650 μg/d vitamin A (as Retinol Equivalents, Table 1), respectively, from a mixture of preformed vitamin A and provitamin A carotenoids (3).
A critical issue with vitamin A is its toxicity (hypervitaminosis A) caused by excessive retinol intake; middle-aged and older adults may be particularly susceptible to vitamin A toxicity (38). Additionally, several prospective cohort studies have reported that higher intakes of retinol are associated with an increased risk of hip fractures in primarily middle-aged and older adults (39), and two RCTs found that high-dose supplementation with β-carotene (alone or combined with retinol) further increased the risk of lung cancer in at-risk populations (e.g. smokers, asbestos-exposed workers) (38). However, a meta-analysis of four RCTs found no effect on lung cancer risk with retinol or β-carotene supplementation in healthy adults (40).
Vitamin D
Vitamin D plays critical roles in calcium metabolism and bone health but is also involved in other health outcomes, such as neurological conditions, autoimmune diseases, NCDs (e.g. type 2 diabetes, cancers), and infections (5, 41). EFSA recommends vitamin D intake of 15 µg/d for adults (3), while the International Osteoporosis Foundation recommends average daily intake of 20–25 µg/d for older adults (41). Older adults may be at particular risk for insufficiency and deficiency due to lower sunlight exposure, reduced ability of the skin to synthesize vitamin D from 7-dehydrocholesterol upon sunlight exposure, and limited consumption of food sources of vitamin D (e.g. fortified milk, oily fish) (5). Across the EU, the percentage of the older population (>64 years) not obtaining adequate amounts of vitamin D is approximately 90% in most areas except Norway, Finland, and Spain (28). A systematic review of 195 studies reported that mean 25(OH)D values for those >65 years of age in the EU were 51.7 nmol/L (42). European League Against Rheumatism (EULAR) and European Federation of National Associations of Orthopaedics and Traumatology (EFORT) recommendations for preventing future fractures in those ≥50 years of age with previous fractures taking anti-osteoporosis drugs include supplementation with 800 IU/d (equivalent to
20 µg/d) vitamin D with adequate calcium intake (1000–1200 mg/d) (43). This recommendation is based on a meta-analysis of RCTs demonstrating a significant reduction in the risk of falling with 700–1000 IU/d (17.5–25 µg/d) vitamin D or 25(OH)D levels of 60–95 nmol/L (44) and a pooled analysis demonstrating a reduced risk of hip fractures with ≥800 IU/d (≥20 µg/d) vitamin D or baseline 25(OH)D levels >60 nmol/L in elderly individuals (≥65 years) (45). A separate meta-analysis of RCTs reported that vitamin D at 482–770 IU/d (12–19 µg/d) reduced the risk for non-vertebral and hip fractures by 20% (44), while a National Osteoporosis Foundation meta-analysis of RCTs reported that vitamin D supplements of 400–800 IU/d (10–20 µg/d) and calcium of 500–1200 mg/d reduced the risk of total and hip fractures by 15% and 30%, respectively (46). Evidence for the effectiveness of vitamin D supplements in reducing the risk of falling is inconsistent, and bolus doses have been shown to increase the risk for falling (47).
It has been suggested that vitamin D can have positive effects on other health outcomes, including autoimmune diseases and CVD, type 2 diabetes, and cancer (48). Deficiencies in 25(OH)D levels may also be associated with increased risk of colorectal and breast cancer, cardiovascular events, and mortality (48). Aggregated evidence from RCTs suggests that vitamin D supplementation could effectively prevent respiratory tract infections (49, 50), which caused 2.8 million deaths worldwide in 2010 (49) and affect the elderly at increased rates (50).
Calcium
Calcium is an essential mineral that is involved in promoting bone health but is also associated with other health outcomes such as controlling blood pressure (5). While EFSA recommends 750 mg/d total calcium intake in adults >50 years of age (3), EULAR/EFORT recommends 1000–1200 mg/d for preventing fractures in those using anti-osteoporosis drugs, with supplementation as necessary (43). EULAR/EFORT also cautions that calcium supplements may produce adverse gastrointestinal and possibly cardiovascular effects (43). Across the EU, the percentage of the older population (>64 years of age) not obtaining adequate amounts of calcium ranges from 48–100% (28).
A meta-analysis of 29 RCTs that evaluated calcium supplementation with or without vitamin D on bone health outcomes in middle-aged and older individuals (≥50 years of age) observed significant reductions in fracture risk and bone loss (51). Concern has been raised about the potential for calcium supplements to increase the risk for CVD, but a long-term study specifically designed to evaluate this potential found no evidence of increased risk in older women (mean age: 75 years) in relation to placebo (52). Another meta-analysis reported that total calcium intake (diet and supplementation) below the tolerable upper intake level (UL) is not associated with an increased risk for CVD (53). However, caution should be taken when recommending calcium supplementation to those already obtaining adequate dietary intake (53).
Vitamin K
Vitamin K describes a group of related fat-soluble vitamins critically involved in coagulation and bone health by activating specific proteins in the bloodstream and bone (3, 5, 54). Vitamin K1 (phylloquinone) is the primary dietary form of vitamin K, which is found in green leafy vegetables and vegetable oils, while vitamin K2 (menaquinone) is primarily found in animal-based or fermented foods (3, 54). EFSA recommends that adults consume 70 µg/d vitamin K (3). The mechanism of action for a class of anticoagulant therapies (e.g. warfarin) used for preventing atrial fibrillation and other cardiovascular events involves vitamin K antagonism; therefore, balancing the dose of these treatments and dietary vitamin K intake should be considered (55). However, studies have provided conflicting results; some show a negative relationship between coagulation stability and vitamin K intake and others suggest that some amount of vitamin K intake is necessary to produce an adequate response (55). A Cochrane database review reported that only one study showed this additive anticoagulant effect, indicating that the current evidence is insufficient to recommend vitamin K for those with unstable response to warfarin (56). Supplemental vitamin K has a strong anticoagulant effect, and consuming high levels of vitamin K-rich foods may interact with anticoagulant treatment (57). Vitamin K1 supplementation combined with calcium and vitamin D3 has also been shown to modestly improve bone mineral content in older non-osteoporotic women; however, these effects were not observed with vitamin K1 alone, suggesting that there may be a synergistic effect of these nutrients (58). Vitamin K2 has also been shown to produce benefits in arterial stiffness and bone mineral density (59, 60).
Vitamin E
Vitamin E (α-tocopherol) is a fat-soluble vitamin with antioxidant properties that are critical for protecting polyunsaturated fatty acids in membrane phospholipids and plasma lipoproteins from oxidative damage (3); it is also involved in immune function (5). α-Tocopherol deficiency causes the development of neurological symptoms (e.g. ataxia) (3). EFSA recommends that adult males and females consume 13 mg/d and 11 mg/d vitamin E, respectively (3), yet those >64 years of age have been shown to consume only between 6.3 and 13.7 mg/d (10). Very few individuals meet intake recommendations for vitamin E through diet alone (5). A meta-analysis of dietary intake studies reported that vitamin E is associated with a dose-dependent reduction in lung cancer risk (61), but another meta-analysis reported no effect on total or cancer-related mortality, aside from a significant reduction in the incidence of prostate cancer when vitamin E was consumed with other nutrients (62). Another meta-analysis of RCTs reported a decreased risk of ischemic stroke but an increased risk for hemorrhagic stroke. Notably, the doses of vitamin E administered substantially exceeded recommended intake levels (63).
Vitamin C
Vitamin C is a water-soluble vitamin with strong reducing and antioxidant properties; it acts as a cofactor in several enzymatic reactions for the synthesis of carnitine, catecholamines, and pro-collagen and for metabolizing cholesterol to bile acids (3). The primary dietary sources of vitamin C include fruit and vegetables and their juices (3). EFSA recommends that adult males and females consume 90 mg/d and 80 mg/d vitamin C, respectively (3). In the EU, the proportion of individuals >64 years of age falling below the average requirement of vitamin C is 4-33% (28).
Population-based studies have shown that plasma vitamin C levels are significantly and inversely related to stroke risk (64). Furthermore, lower vitamin C levels have been linked with a greater risk for Alzheimer’s disease (65, 66). Meta-analyses of RCTs have found that vitamin C supplementation significantly reduces both systolic and diastolic blood pressure (67), improves endothelial function and vasodilation in individuals with cardiometabolic risk factors (68), and decreases risk for lung cancer (69) and age-related cataracts (70).
Magnesium
Magnesium is an essential mineral for many enzymatic reactions involved in the synthesis of carbohydrates, lipids, nucleic acids, and proteins, and serves in various neurological and cardiovascular functions, including regulating blood pressure (3, 5). Magnesium is primarily found in muscle tissues and is an important component of bone (3, 5). Magnesium occurs naturally in a number of food items, including nuts, whole grains, seafood, fruit, and vegetables (3). EFSA recommends that adult men and women consume 350 mg/d and 300 mg/d of magnesium, respectively (3). There are few clear indications of magnesium inadequacies due to its various metabolic effects, and serum magnesium concentration as an indicator of status is questionable since there are no reliable biomarkers for magnesium body status (3).
Meta-analyses of prospective cohort and observational studies conducted primarily in middle-aged individuals have reported an association between higher circulating magnesium concentrations and decreased CVD risk (71), a significant inverse relationship between dietary magnesium intake and risk of metabolic syndrome (72), and a relationship between higher magnesium intake and reductions in colorectal cancer (73). Meta-analyses of RCTs that evaluated magnesium supplementation on diabetes-related outcomes reported improvements in insulin resistance (74) and fasting glucose levels (75) and significant reductions in systolic and diastolic blood pressure (76), including in individuals with insulin resistance, pre-diabetes, and other NCDs (77).
Potassium
Potassium is the primary intracellular cation responsible for maintaining fluid and electrolyte balance in the body and, hence, proper nerve conduction, muscle contraction, blood volume, and blood pressure (3, 5). Potassium occurs naturally in all foods but is particularly represented in root vegetables, fruit, whole grains, coffee, and dairy (3). EFSA recommends that adults consume 3500 mg/d of potassium, but these values can vary by country (3). Insufficient potassium intake causes hypertension and increases the risk of CVD, kidney stones, and osteoporosis (5).
A meta-analysis of prospective cohort studies reported that higher dietary potassium intake reduced the risk of stroke, which the authors attributed to reduced blood pressure (78). Although a 2006 Cochrane database review did not find substantial support for potassium supplementation for hypertension (79), a more recent meta-analysis of RCTs reported that potassium supplementation reduced systolic and diastolic blood pressure in a dose-dependent manner in hypertensive individuals (80).
Ensuring adequate nutrition in middle-aged and older adults
There is a robust body of evidence suggesting that a whole-diet approach not only lowers mortality from NCDs, but also positively impacts physical and cognitive functioning, mental health, and quality of life in older adults (81). However, too few high-quality studies have evaluated these outcomes to make clear recommendations. In addition to the nutritional benefits of a healthful diet, there are psychological benefits to eating that should not be overlooked (82). Increasing contact with professionals, identifying barriers, developing problem-solving techniques, and setting appropriate goals can improve the outcomes of dietary interventions in middle-aged and older individuals (83). Nutrition education programs have been shown to be effective for improving the nutritional status of older adults, particularly when they include specific interventions or multiple sessions (84).
Higher adherence to a Mediterranean Diet has been found to be significantly and inversely related to overall mortality in adults >65 years of age (85) and to reduced all-cause, CVD- and cancer-related mortality (86). Furthermore, a multivitamin/multimineral supplement (MVMS) that provides most micronutrients in recommended amounts can provide nutritional support for those who are unable to reach adequate micronutrient intake levels from their habitual diet, particularly for older individuals who often experience malnutrition with advancing age (87).
Healthcare providers commonly recommend MVMS to older adults, and while MVMS are generally safe (88), their benefits for improving health-related outcomes have been difficult to conclusively demonstrate (89). The Physicians’ Health Study II (PHS II) observed no reduction in the risk for developing CVD (90), but there was a significant 8% reduction in the risk of all types of cancer in this middle-aged and older male population (≥50 years of age) taking a daily MVMS for a mean duration of 11 years (91). The reduction in cancer risk was 12% when excluding prostate cancer from the analysis, and even greater (27%) in men with a baseline history of cancer (91). Despite its long duration and large sample size involving more than 14,000 male physicians, PHS II was insufficiently powered to detect statistically significant effects of MVMS on any individual type of cancer (91).
PHS II also found a significant 9% reduction in total age-related cataracts and an 11% reduction in cataract surgery (92). According to a Cochrane database review, use of an MVMS with antioxidant vitamins and minerals may delay the progression of age-related macular degeneration (AMD) (93). Lutein and zeaxanthin, which are sometimes added to MVMS formulations, also seem to be beneficial for the management of AMD (94). A meta-analysis of eight RCTs utilizing lutein and zeaxanthin supplementation showed improvements in visual acuity and contrast sensitivity in subjects with AMD (95).
Conflicting evidence on cognition has been observed in older adults who use MVMS. One clinical trial in middle-aged and older men reported improvements in episodic memory, including contextual recognition (96), while another trial in healthy middle-aged and older adults reported no benefit in cognitive task performance (97). Both of these studies reported that MVMS use improved some health-related biomarkers (i.e. C-reactive protein, liver function, and vitamin B6 and B12 blood levels, cholesterol, and homocysteine levels) (96, 97). A meta-analysis of 10 RCTs in primarily middle-aged and older adults reported that MVMS modestly improved some aspects of memory (98). An MVMS formulated with folic acid and vitamins B6 and B12 was shown to improve plasma homocysteine concentrations in an RCT in individuals ≥50 years of age (99), and another study in older individuals being treated with metformin demonstrated that use of an MVMS can reduce the risk for metformin-mediated deficiencies in vitamin B12 levels (100).
It is important that an MVMS include nutrients only in amounts that approximate reference intake values, and middle-aged and older adults who decide to use an MVMS should be aware that using additional single-nutrient supplements could result in a total intake exceeding the UL of these nutrients, increasing the risk of adverse health outcomes (87). Furthermore, there may be some individual exceptions to consider for these populations. For example, the typical dose of calcium commonly used in an MVMS may not be sufficient to promote bone health; therefore, dietary intake should be considered (89).
Conclusion
Data from RCTs suggest that dietary interventions and, when warranted, supplementation with MVMS can be used to reduce the risk of experiencing nutritional deficiencies and inadequacies that are detrimental to the health of middle-aged and older adults. For individuals who do not consume adequate protein, fiber, omega-3 fatty acids, and micronutrients from their habitual diet, supplementation may be required. The individual’s medical history, medication use, dietary patterns, and other risk factors should be considered when recommending dietary supplements.
Funding
Medical writing support was provided by Dennis Stancavish of Peloton Advantage, LLC, and was funded by Pfizer.
Role of the Sponsor
The sponsor was involved in the review and approval of the manuscript.
Conflicts of Interest
Susanne Bügel has received research grants from the Software AG Foundation and ERASMUS+. She is a board member of Food, Quality and Health and the Federation of European Nutrition Societies and as such receives travel support for meetings supported by these organizations. Balz Frei is the past recipient of multiple grants from the US National Institutes of Health and currently serves as a consultant for Pfizer Consumer Healthcare and DSM Nutritional Products.
References
1. World Health Organization. The European health report 2012: Charting the way to well-being. 2013. World Health Organization, Geneva, Switzerland.
2. World Health Organization Regional Office for Europe. Strategy and action plan for healthy ageing in Europe, 2012-2020. 2012. World Health Organization Regional Office for Europe, Copenhagen, Denmark.
3. Online document. European Food Safety Authority. Dietary reference values for nutrients, summary report. European Food Safety Authority. 2017. https://www.efsa.europa.eu/sites/default/files/2017_09_DRVs_summary_report.pdf. Accessed November 21, 2017.
4. Favaro-Moreira NC, Krausch-Hofmann S, Matthys C, et al. Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data. Adv Nutr 2016;7:507-522.
5. National Academies of Sciences Engineering Medicine. Meeting the dietary needs of older adults: Exploring the impact of the physical, social, and cultural environment: Workshop summary. 2016. National Academies Press, Washington, DC.
6. Higdon J, Drake VJ. Drug-nutrient interactions. 2012. Georg Thieme Verlag, Stuttgart, Germany.
7. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr 2015;113:1195-1206.
8. Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A 2006;103:17589-17594.
9. Lorenzo-Lopez L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodriguez-Villamil JL, Millan-Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr 2017;17:108.
10. Elmadfa I, Meyer A, Nowak V, et al. European nutrition and health report 2009. Forum Nutr 2009;62:1-405.
11. Nowson C, O’Connell S. Protein requirements and recommendations for older people: A review. Nutrients 2015;7:6874-6899.
12. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748-759.
13. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14:542-559.
14. Pedersen AN, Cederholm T. Health effects of protein intake in healthy elderly populations: a systematic literature review. Food Nutr Res 2014;58.
15. Liao CD, Tsauo JY, Wu YT, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr 2017;106:1078-1091.
16. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999-3058.
17. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30-42.
18. Streppel MT, Arends LR, van ‘t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165:150-156.
19. Post RE, Mainous AG 3rd, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med 2012;25:16-23.
20. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769-1818.
21. Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc 2017;92:15-29.
22. Wang C, Xiong B, Huang J. The role of omega-3 polyunsaturated fatty acids in heart failure: a meta-analysis of randomised controlled trials. Nutrients 2016;9.
23. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage 2012;20:382-387.
24. Jiao J, Li Q, Chu J, Zeng W, Yang M, Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;100:1422-1436.
25. Yurko-Mauro K, Alexander DD, Van Elswyk ME. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One 2015;10:e0120391.
26. Forbes SC, Holroyd-Leduc JM, Poulin MJ, Hogan DB. Effect of nutrients, dietary supplements and vitamins on cognition: a systematic review and meta-analysis of randomized controlled trials. Can Geriatr J 2015;18:231-245.
27. Porter K, Hoey L, Hughes CF, Ward M, McNulty H. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients 2016;8:725.
28. Roman Viñas B, Barba LR, Ngo J, et al. Projected prevalence of inadequate nutrient intakes in Europe. Ann Nutr Metab 2011;59:84-95.
29. Russell RM. Factors in aging that effect the bioavailability of nutrients. J Nutr 2001;131:1359s-1361s.
30. Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc 2016;5.
31. Homocysteine Lowering Trialists’ Collaboration Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 2005;82:806-812.
32. van Wijngaarden JP, Swart KM, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr 2014;100:1578-1586.
33. Fan C, Yu S, Zhang S, Ding X, Su J, Cheng Z. Association between folate intake and risk of head and neck squamous cell carcinoma: An overall and dose-response PRISMA meta-analysis. Medicine (Baltimore) 2017;96:e8182.
34. Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297:2351-2359.
35. Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. Am J Med 2010;123:522-527.
36. Walker JG, Batterham PJ, Mackinnon AJ, et al. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms–the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr 2012;95:194-203.
37. Sherwin JC, Reacher MH, Dean WH, Ngondi J. Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations. Trans R Soc Trop Med Hyg 2012;106:205-214.
38. Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr 2000;71:878-884.
39. Wu AM, Huang CQ, Lin ZK, et al. The relationship between vitamin A and risk of fracture: meta-analysis of prospective studies. J Bone Miner Res 2014;29:2032-2039.
40. Cortes-Jofre M, Rueda JR, Corsini-Munoz G, Fonseca-Cortes C, Caraballoso M, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev 2012;10:CD002141.
41. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 2010;21:1151-1154.
42. Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr 2014;111:23-45.
43. Lems WF, Dreinhofer KE, Bischoff-Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 2017;76:802-810.
44. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:551-561.
45. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 2012;367:40-49.
46. Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation [published erratum and additional analyses appear in Osteoporos Int. 2016;27(8):2643-2646] Osteoporos Int 2016;27:367-376.
47. Gallagher JC. Vitamin D and falls – the dosage conundrum. Nat Rev Endocrinol 2016;12:680-684.
48. Bruyere O, Cavalier E, Souberbielle JC, et al. Effects of vitamin D in the elderly population: current status and perspectives. Arch Belg Sante Publ 2014;72:32.
49. Bergman P, Lindh AU, Bjorkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2013;8:e65835.
50. Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis 2008;46:1582-1588.
51. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan. A Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007;370:657-666.
52. Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res 2011;26:35-41.
53. Chung M, Tang AM, Fu Z, Wang DD, Newberry SJ. Calcium intake and cardiovascular disease risk: An updated systematic review and meta-analysis. Ann Intern Med 2016;165:856-866.
54. Grober U, Reichrath J, Holick MF, Kisters K Vitamin K: an old vitamin in a new perspective. Dermatoendocrinol 2014;6:e968490.
55. Violi F, Lip GY, Pignatelli P, Pastori D Interaction between dietary vitamin K intake and anticoagulation by vitamin K antagonists: Is it really true?: a systematic review. Medicine (Baltimore) 2016;95:e2895.
56. Mahtani KR, Heneghan CJ, Nunan D, Roberts NW. Vitamin K for improved anticoagulation control in patients receiving warfarin. Cochrane Database Syst Rev 2014;5:CD009917.
57. Bugel SG, Spagner C, Poulsen SK, Jakobsen J, Astrup A. Phylloquinone content from wild green vegetables may contribute substantially to dietary intake. Can J Agric Crops 2016;1:83-88.
58. Bolton-Smith C, McMurdo ME, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 2007;22:509-519.
59. Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int 2013;24:2499-2507.
60. Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost 2015;113:1135-1144.
61. Zhu YJ, Bo YC, Liu XX, Qiu CG. Association of dietary vitamin E intake with risk of lung cancer: a dose-response meta-analysis. Asia Pac J Clin Nutr 2017;26:271-277.
62. Alkhenizan A, Hafez K. The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trials. Ann Saudi Med 2007;27:409-414.
63. Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010;341:c5702.
64. Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr 2008;87:64-69.
65. Harrison FE. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J Alzheimers Dis 2012;29:711-726.
66. de Wilde MC, Vellas B, Girault E, Yavuz AC, Sijben JW. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimers Dement (N Y) 2017;3:416-431.
67. Juraschek SP, Guallar E, Appel LJ, Miller ER, III. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;95:1079-1088.
68. Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014;235:9-20.
69. Luo J, Shen L, Zheng D. Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Sci Rep 2014;4:6161.
70. Wei L, Liang G, Cai C, Lv J. Association of vitamin C with the risk of age-related cataract: a meta-analysis. Acta Ophthalmol 2016;94:e170-176.
71. Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2013;98:160-173.
72. Ju SY, Choi WS, Ock SM, Kim CM, Kim DH. Dietary magnesium intake and metabolic syndrome in the adult population: dose-response meta-analysis and meta-regression. Nutrients 2014;6:6005-6019.
73. Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. Eur J Clin Nutr 2012;66:1182-1186.
74. Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Guerrero-Romero F. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res 2016;111:272-282.
75. Veronese N, Watutantrige-Fernando S, Luchini C, et al. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: a systematic review and meta-analysis of double-blind randomized controlled trials. Eur J Clin Nutr 2016;70:1354-1359.
76. Zhang X, Li Y, Del Gobbo LC, et al. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016;68:324-333.
77. Dibaba DT, Xun P, Song Y, Rosanoff A, Shechter M, He K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2017;106:921-929.
78. D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol 2011;57:1210-1219.
79. Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev 2006;3:CD004641.
80. Poorolajal J, Zeraati F, Soltanian AR, Sheikh V, Hooshmand E, Maleki A. Oral potassium supplementation for management of essential hypertension: A meta-analysis of randomized controlled trials. PLoS One 2017;12:e0174967.
81. Milte CM, McNaughton SA. Dietary patterns and successful ageing: a systematic review. Eur J Nutr 2016;55:423-450.
82. Plastow NA, Atwal A, Gilhooly M. Food activities and identity maintenance in old age: a systematic review and meta-synthesis. Aging Ment Health 2015;19:667-678.
83. Lara J, Evans EH, O’Brien N, et al. Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomised controlled trials. BMC Med 2014;12:177.
84. Raffaele B, Matarese M, Alvaro R, De Marinis MG. Health-promotion theories in nutritional interventions for community-dwelling older adults: a systematic review. Ann Ist Super Sanita 2017;53:146-151.
85. McNaughton SA, Bates CJ, Mishra GD. Diet quality is associated with all-cause mortality in adults aged 65 years and older. J Nutr 2012;142:320-325.
86. Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881-889.
87. Biesalski HK, Tinz J Multivitamin/mineral supplements: rationale and safety – a systematic review. Nutrition 2017;33:76-82.
88. Macpherson H, Pipingas A, Pase MP. Multivitamin-multimineral supplementation and mortality: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2013;97:437-444.
89. Buhr G, Bales CW. Nutritional supplements for older adults: review and recommendations-part I. J Nutr Elder 2009;28:5-29.
90. Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2012;308:1751-1760.
91. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men. The Physicians’ Health Study II randomized controlled trial. JAMA 2012;308:1871-1880.
92. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology 2014;121:525-534.
93. Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 2017;7:CD000254.
94. Scripsema NK, Hu DN, Rosen RB. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J Ophthalmol 2015;2015:865179.
95. Liu R, Wang T, Zhang B, et al. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;56:252-258.
96. Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol 2012;27:370-377.
97. Harris E, Macpherson H, Pipingas A. Improved blood biomarkers but no cognitive effects from 16 weeks of multivitamin supplementation in healthy older adults. Nutrients 2015;7:3796-3812.
98. Grima NA, Pase MP, Macpherson H, Pipingas A. The effects of multivitamins on cognitive performance: a systematic review and meta-analysis. J Alzheimers Dis 2012;29:561-569.
99. McKay DL, Perrone G, Rasmussen H, Dallal G, Blumberg JB. Multivitamin/mineral supplementation improves plasma B-vitamin status and homocysteine concentration in healthy older adults consuming a folate-fortified diet. J Nutr 2000;130:3090-3096.
100. Kancherla V, Garn JV, Zakai NA, et al. Multivitamin use and serum vitamin B12 concentrations in older-adult metformin users in REGARDS, 2003-2007. PLoS One 2016;11:e0160802.